The U.S. Centers for Disease Control and Prevention (CDC) is considering the use of AI to back up its tuberculosis (TB) screening program for immigrants and refugees.
In an article published September 30 in PLOS Digital Health, scientists at the agency noted that they have few methods currently for ensuring that the half a million x-rays they receive every year from physicians overseas have been interpreted correctly.
“To make these assessments more efficient, we developed a machine-learning algorithm that can reliably detect signs of tuberculosis in the x-rays. In testing, the algorithm worked well on a variety of datasets, suggesting it will be a good tool for supporting these important quality control efforts,” noted lead author Scott Lee, PhD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases in Atlanta.
After COVID-19, TB is the second leading cause of death from infectious disease in the world. The U.S. has relatively low rates – about 2.5 cases per 100,000 members of the population in 2022 – but immigrants, refugees, and other migrants seeking entry into the U.S. often come from areas where the background rates are much higher, the authors explained.
To help prevent the disease from being imported, the CDC runs an overseas screening program through its Division for Global Migration Health (DGMH) in which every applicant aged 15 years or older undergoes a chest x-ray. The DGMH conducts ad-hoc quality control assessments to make sure the x-rays have been interpreted according to the program’s standards.
In the study, the scientists culled a dataset of 152,012 digital chest x-rays from the program and trained deep-learning models to perform three tasks: identify abnormal radiographs, identify abnormal radiographs suggestive of tuberculosis, and identify specific findings such as cavities or infiltrates in abnormal radiographs.
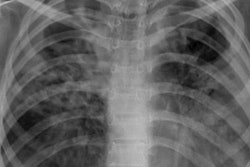
 XRAI (left) and GradCAM (right) heatmaps for true positive images. The original radiographs are on the left, the Grad-CAM activations and heatmaps are in the middle, and the XRAI activations and overlays are on the right. For the XRAI overlays, only regions reaching the 70th percentile of activation strength are shown. Images available under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of PLOS Digital Health.
XRAI (left) and GradCAM (right) heatmaps for true positive images. The original radiographs are on the left, the Grad-CAM activations and heatmaps are in the middle, and the XRAI activations and overlays are on the right. For the XRAI overlays, only regions reaching the 70th percentile of activation strength are shown. Images available under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of PLOS Digital Health.
On an internal dataset of 8,000 images (50% abnormal), the models performed well both in identifying abnormalities suggestive of TB (area under the curve [AUC] of 0.97), and in estimating sample-level counts of the same (-2% absolute percentage error).
On an external dataset also of 8,000 images (50% abnormal), the models performed similarly well in identifying both generic abnormalities (AUCs ranging from 0.89 to 0.92) and those suggestive of TB (AUCs from 0.94 to 0.99).
“We can imagine running the models on batches of incoming images and comparing their number of positive calls to the number of reported abnormal radiographs, raising an alert when the difference in counts exceeds a predefined threshold,” the group wrote.
However, considerably more studies will be needed before deploying the technology, the authors noted.
“Although some technical innovation may be required to make this approach feasible, it may well improve our ability to predict tuberculosis disease, especially in low-resourced settings where access to trained radiologists is limited, and thus seems well worth pursuing,” the group concluded.
The full article can be found here.