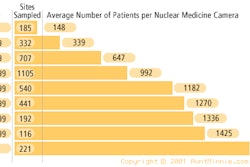
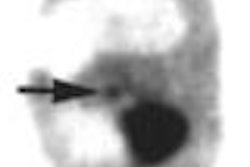
The nuclear medicine community may be convinced of PET's value in diagnosing Alzheimer's disease (AD), but the U.S. government isn't sure enough to pay for it. On Thursday a Medicare panel recommended that the Centers for Medicare and Medicaid Services not cover use of FDG-PET in the diagnosis and management of the disorder.
According to the Reston, VA-based Society of Nuclear Medicine, the Medicare Coverage Advisory Committee of CMS voted unanimously against coverage, saying the evidence does not adequately demonstrate "that PET has clinical benefit in evaluating patients" with possible or probable AD or with mild cognitive impairment as defined by the current American Academy of Neurology guidelines.
In evaluating the application, the Agency for Health Care Research and Quality (AHRQ) used the current "treat all" AAN guideline for AD to measure the improvement of outcomes by using PET. By this measure, the more definitive diagnosis offered by using PET is unnecessary, according to the SNM.
Dr. Peter Conti presented testimony in support of the application on behalf of the SNM.
By AuntMinnie.com staff writers
January 11, 2002
Related Reading
CMS tackles FDG-PET reimbursement for Alzheimer’s, January 10, 2002
PET scanning plus new molecular probe detects early Alzheimer's disease in vivo, January 10, 2002
PET payment cut will force efficiency, procedure volume gains, December 6, 2001
Wagner taps PET Alzheimer’s study as SNM 2001’s Image of the Year, June 6, 2001
Copyright © 2002 AuntMinnie.com