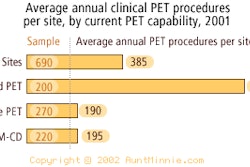
PET manufacturer Positron has received Food and Drug Administration 510(k) clearance for its Posicam HZ, Posicam HZL, and mPower PET systems. The pre-market notification covered iterative reconstruction (IR), post-injection transmission acquisition (PITA), and compliance with part 10 of the DICOM Standard, according to the Houston-based vendor.
By AuntMinnie.com staff writersAugust 19, 2002
Related Reading
Positron revenues jump, losses continue, August 19, 2002
Positron sells two mPower scanners, July 9, 2002
Positron trims loss, ships scanner in Q1, May 17, 2002
Positron issues "going concern" warning, April 2, 2002
Positron partners with HealthCare Concepts, January 14, 2002
Copyright © 2002 AuntMinnie.com