Researchers from the Duke University Medical Center in Durham, NC, are heralding early success with the isotope astatine-211 (At-211) in destroying residual cancer cells in the brain, while sparing healthy cells, and extending the lives of cancer patients.
In the first-ever clinical trial of the isotope, the researchers found that administering the alpha-particle-emitting At-211 ch81C6 into the surgically created resection cavity of patients with recurrent central nervous system tumors after resection is feasible. They also found that toxicity to patients who undergo the treatment is minimal.
The study was supported by grants from the National Institutes of Health and published in the Journal of Nuclear Medicine (January 2008, Vol. 49:1, pp. 30-38). It included 18 patients with recurrent brain tumors treated at Duke University Medical Center between April 1998 and June 2001.
The group was comprised of nine women and nine men, with a median age of 50 years, ranging from 27 to 76 years old. Fourteen patients had glioblastoma multiforme, three patients had anaplastic oligodendroglioma, and one patient had anaplastic astrocytoma.
Eligible patients had a confirmed histologic diagnosis of recurrent supratentorial primary malignant tumors and were candidates for surgical resection. All patients received external beam radiotherapy before administration of At-211 ch81C6; eight patients had received prior chemotherapy.
Toxicity monitoring
The patients were monitored for toxicity and survival after receiving the isotope, with initial follow-up occurring in the first month after treatment. Chemotherapy also was prescribed for one year, beginning approximately four weeks after At-211 ch81C6 administration.
Patient monitoring continued with re-evaluation every eight to 12 weeks during chemotherapy. The watch continued every three months during the first year, every four months in the second year, and twice a year in the third year and beyond.
The pilot study showed promising results in that the administration of At-211 ch81C6 into surgically created resection cavities with recurrent central nervous system tumors after resection was feasible. In addition, no patient experienced dose-limiting toxicity after the administration of doses up to 347 MBq.
The therapy also extended patients' survival rates by one to three years, with a median time of 54.1 weeks. Without treatment, patients have a life expectancy of approximately six months.
Isotope upside
A potential advantage of using shorter-range alpha-particle emitters, such as At-211 ch81C6, to treat brain tumors is that "it might be possible to achieve efficacy similar to that achieved with (beta)-particle emitters, but with a lower toxicity for normal brain regions in the vicinity of the (surgically created resection cavity)," the researchers noted.
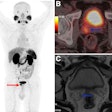
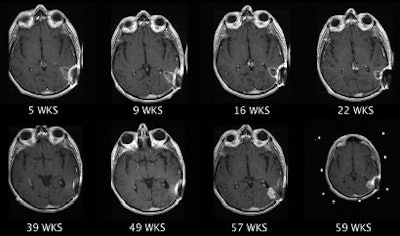 |
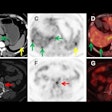
| Clinical MRI images of a patient after At-211 ch81C6 therapy. After At-211 ch81C6 administration, surgically created resection cavity rim enhancement gradually became more prominent, as surgically created resection cavity retracted. Focal nodular enhancement noted 57 weeks after At-211 ch81C6 administration was confirmed to the recurrent anaplastic oligodendroglioma. Image courtesy of Duke University Medical Center and reprinted with permission from "Clinical Experience with α-Particle-Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6," Michael R. Zalutsky, David A. Reardon, Gamal Akabani, R. Edward Coleman, Allan H. Friedman, Henry S. Friedman, Roger E. McLendon, Terence Z. Wong, and Darell D. Bigner. January 2008 Journal of Nuclear Medicine. |
It is "encouraging that eight of 14 patients with recurrent (glioblastoma multiforme) survived for one year and that two patients survived for nearly three years after receiving 144 MBq of At-211 ch81C6," they added. "It is also encouraging that the median survival time of 52 weeks observed in the present study compared favorably with the median survival times of 23 (weeks) and 31 weeks reported for patients with recurrent (glioblastoma multiforme) treated with best care, plus placebo and carmustine polymers, respectively."
Study co-author Dr. Michael Zalutsky, professor of radiology and biomedical engineering at Duke University Medical Center, sees the ability to kill cancer cells in the brain without damaging the neurological system that controls a patient's brain function as an important step in treating this form of cancer and others, such as ovarian and breast cancers.
With the encouraging results, Duke researchers advocate additional evaluation of At-211 ch81C6 for patients with central nervous system tumors, with studies extending to multiple surgically created resection cavity dose administration schedules and the use of At-211 ch81C6 as part of a radiotherapeutic "cocktail" containing a beta-emitter to modulate the dose profile and intrathecal administration for patients with leptomeningeal carcinomatosis.
By Wayne Forrest
AuntMinnie.com staff writer
February 21, 2008
Related Reading
Presurgical fMRI for tumor resection: Are we there yet? May 21, 2007
Functional MRI allows more aggressive brain tumor treatment, August 31, 2006
PET trumps MRI for brain cancer chemo response, July 7, 2006
Copyright © 2008 AuntMinnie.com