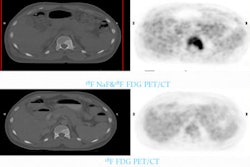
Swiss researchers have found evidence that PET imaging may detect metabolic changes following treatment with the protein kinase inhibitor vandetanib, and help in the evaluation of therapy response, according to a study in the February issue of the Journal of Nuclear Medicine.
Vandetanib, which currently is in clinical trials, is designed to inhibit the function of the RET (rearranged-during-transfection protein) proto-oncogene and other protein kinases involved in the development and progression of cancer. Lead study author Martin A. Walter, MD, said the trials are assessing the effectiveness of vandetanib by monitoring changes in tumor size (JNM, Vol. 52:2, pp. 231-240). Walter is with University Hospital in Bern, Switzerland, and the University of California, Los Angeles.
Walter and colleagues first examined medullary thyroid cancer cells to create an in vitro model. After cultivation, the cells were treated with vandetanib, and changes in their metabolic profile were monitored successfully by transcriptional profiling and radiotracer uptake studies.
Using the same untreated cells, the researchers then created an in vivo model by injecting mice with the cancerous cells and treating them with vandetanib. Small animal PET/CT also was performed and was found to reproduce the in vitro findings of metabolic activity after three days.
In addition, the study included a 43-year-old patient with biopsy-proven metastasized medullary thyroid cancer who was treated with 300 mg of vandetanib per day. After fasting for at least four hours, the patient intravenously received 300 MBq of FDG, followed by a whole-body PET scan (Siemens Healthcare, Erlangen, Germany).
FDG-PET scans were taken at 12 and 24 weeks after treatment to determine the metabolic response to vandetanib, consistent with the in vitro and in vivo samples.
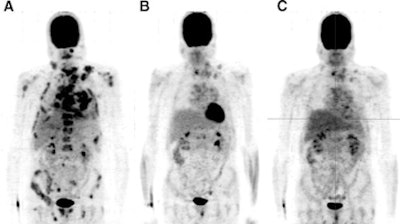 |
| FDG-PET scans of a 43-year-old man with metastasized medullary thyroid cancer before (A) and at 12 (B) and 24 (C) weeks after start of treatment with 300 mg of vandetanib per day. Image courtesy of the Journal of Nuclear Medicine. |
Results showed that glucose levels were within the reference range at each scanning time point. The maximum standardized uptake value (SUV) was measured in 10 reference lesions at the baseline scan and at each follow-up scan. The SUV dropped from 8.1 ± 1.5 at baseline to 4.0 ± 1.3 after 12 weeks and 3.4 ± 1.2 after 24 weeks of treatment.
With increasing treatment options, Walter and colleagues noted that diligent patient selection is necessary to ensure targeted therapy is administered to people most likely to benefit from treatment. "The identification of markers of treatment efficacy is a key factor for the success of these novel treatment approaches," the authors wrote.
By Wayne Forrest
AuntMinnie.com staff writer
February 2, 2011
Related Reading
Thyroid cancer risk from ped neck CT shouldn't be generalized, December 24, 2010
Radiation ups pediatric thyroid risk, December 17, 2010
Screening for thyroid cancer worthwhile in some childhood cancer survivors, December 29, 2008
Copyright © 2011 AuntMinnie.com