Nuclear medicine departments now have a second reliable radiopharmaceutical to consider with PET/CT when staging for prostate cancer, according to a study in the April issue of the Journal of Nuclear Medicine.
Israeli researchers found F-18 prostate-specific membrane antigen (PSMA)-1007 effective for detecting malignant prostate cancer lesions. They believe the radiopharmaceutical has a longer half-life and potentially better spatial resolution than gallium-68 (Ga-68) PSMA-11 (JNM, April 2020, Vol. 61:4, pp. 527-532).
"Ga-68 has a half-life of only 68 minutes, compared with 110 minutes for F-18, and emits a higher-energy positron than F-18," said study co-author Dr. Einat Even-Sapir, PhD, from Tel Aviv Sourasky Medical Center, in a statement. F18-PSMA-1007 also offers "favorable pharmacokinetics" and is currently available.
The researchers prospectively compared the diagnostic accuracy of F-18 PSMA-1007 to that of Ga-68-PSMA-11 in PET/CT scans in a group of16 patients who were scheduled to undergo a radical prostatectomy for newly diagnosed intermediate- or high-risk prostate cancer. The results from both radiopharmaceuticals then were compared with histopathologic findings, which were the gold standard.
While radiolabeled PSMA-avid lesions in the prostate were identified in all 16 patients with almost perfect agreement between the two tracers regarding tumor location F-18-PSMA-1007 identified additional foci in four patients. Three lesions (75%) were confirmed as areas of prostate cancer, while the fourth lesion (25%) was deemed chronic prostatitis.
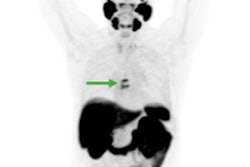
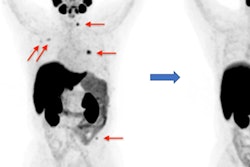
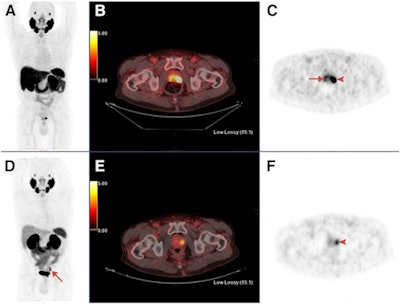 Maximum-intensity projections (MIP), transaxial fusion, and PET images of F-18-PSMA-1007 (A-C) and Ga-68-PSMA-11 (D-F) PET/CT scans from a 67-year-old patient with a Gleason score of 8 and prostate-specific antigen (PSA) score of 4.9 ng/mL. Marked uptake is seen in urinary bladder and left ureter (arrow) on MIP image of Ga-68-PSMA-11 (D), compared with nearly negligible F-18-PSMA-1007 urinary excretion (A). Dominant lesion in left prostatic lobe is evident on both scans (arrowheads). However, a second lesion is seen in right lobe only on 18F-PSMA-1007 scan (arrow in C), later verified on pathology as true malignant lesion. Images courtesy of the Journal of Nuclear Medicine.
Maximum-intensity projections (MIP), transaxial fusion, and PET images of F-18-PSMA-1007 (A-C) and Ga-68-PSMA-11 (D-F) PET/CT scans from a 67-year-old patient with a Gleason score of 8 and prostate-specific antigen (PSA) score of 4.9 ng/mL. Marked uptake is seen in urinary bladder and left ureter (arrow) on MIP image of Ga-68-PSMA-11 (D), compared with nearly negligible F-18-PSMA-1007 urinary excretion (A). Dominant lesion in left prostatic lobe is evident on both scans (arrowheads). However, a second lesion is seen in right lobe only on 18F-PSMA-1007 scan (arrow in C), later verified on pathology as true malignant lesion. Images courtesy of the Journal of Nuclear Medicine."In view of the near-equal performance of the two tracers, this preliminary study suggests the routine use of F-18-PSMA-1007 in lieu of Ga-68-PSMA-11 for staging prostate cancer patients," added study co-author Dr. Jonathan Kuten from Tel Aviv Sourasky Medical Center. "Clinicians can use either radiotracer based on availability."