A new PET tracer designed to improve the detection of tau deposits in key brain regions of patients suspected of Alzheimer's disease could greatly advance accurate diagnoses, according to a proof-of-concept study published in the June issue of the Journal of Nuclear Medicine.
The research was conducted and funded by Berlin-based Life Molecular Imaging (LMI) for its F-18-PI-2620 PET radioligand, which achieved greater uptake in targeted areas associated with Alzheimer's disease in patients suspected of having the illness, compared with healthy controls. The radiopharmaceutical also didn't collect in pockets that are not relevant to cognitive dysfunction.
"The present study showed accumulation of F-18-PI-2620 in regions known to have tau deposition in [amyloid-beta-positive] clinically probable Alzheimer's subjects," wrote the authors, led by Andre Mueller, head principal of R&D at LMI. "Healthy control subjects showed very low F-18-PI-2620 accumulation, and Alzheimer's subjects could be clearly distinguished."
Like other PET tracers, F-18-PI-2620 is designed to bind to tau deposits, which are linked to Alzheimer's disease and other neurodegenerative disorders. Other tau tracers, however, have shown a tendency to bind in brain regions that are not associated with cognitive dysfunction, such as the basal ganglia and choroid plexus. This errant uptake can hamper accurate diagnoses.
This past January, LMI (formerly known as Piramal Imaging) unveiled encouraging results on studies of its PET neuroimaging tracers, which included the combination of its own florbetaben F-18 injection (Neuraceq) with F-18-PI-2620 to detect tau pathology in patients with Alzheimer's disease, as well as people with progressive supranuclear palsy and corticobasal syndrome.
In the current study, LMI, which is collaborating with Swiss biopharmaceutical company AC Immune, sought to further prove that F-18-PI-2620 can better target Alzheimer's-lined tau (JNM, June 2020, Vol. 61:6, pp. 911-919).
To do so, Mueller and colleagues enlisted 12 patients with Alzheimer's disease (mean age, 68.5 ± 9.8 years) and 10 health control subjects (mean age, 59.2 ± 7.8 years) who met the study criteria. All of the participants underwent continuous F-18-PI-2620-PET scans (Ecat Exact HR1, Siemens Healthineers) for up to 180 minutes over two imaging sessions after injection of one dose of F-18-PI-2620 (338.7 ± 20.9 MBq; range, 262.7-359.8 MBq) followed by a 10-mL saline flush.
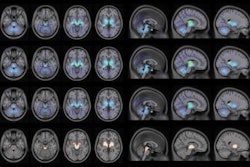
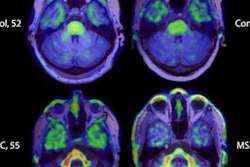
Their visual analyses of the resulting tau PET images showed F-18-PI-2620 uptake in the cortical regions of patients with Alzheimer's disease, particularly in the temporal and parietal lobes, precuneus, and posterior cingulate cortex. By comparison, there was no such uptake in cerebral brain regions of healthy control subjects, which allowed for their differentiation from patients with Alzheimer's disease, the authors noted.
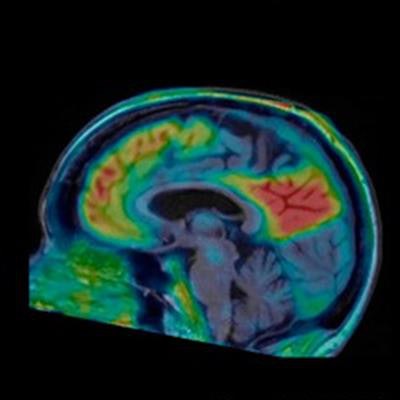
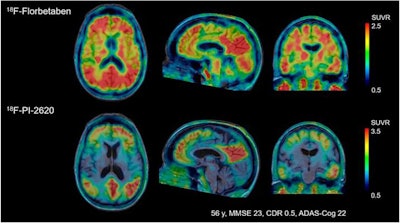 Images compare florbetaben amyloid-PET (top row) and F-18-PI-2620 tau PET (bottom row) obtained in same subject who had a clinical dementia rating (CDR) of 5. Images were normalized to cerebellar gray matter and coregistered to subject's MR images. Images courtesy of Journal of Nuclear Medicine.
Images compare florbetaben amyloid-PET (top row) and F-18-PI-2620 tau PET (bottom row) obtained in same subject who had a clinical dementia rating (CDR) of 5. Images were normalized to cerebellar gray matter and coregistered to subject's MR images. Images courtesy of Journal of Nuclear Medicine."This is an improvement over the first-generation tau agents, in which off-target binding may confound visual and quantitative assessments and may limit the ability to specifically image tau pathology, such as in the mesial-temporal regions," Mueller and colleagues added.
Both sets of subjects also showed "high initial brain uptake" approximately 5 minutes after injection and "rapid clearance" of the tracer from nontargeted regions that did not contain tau deposits, although the "washout was slower in Alzheimer's subjects than in healthy controls in areas where an accumulation of neurofibrillary tangles can be expected," the authors wrote.
Another promising finding was no adverse or clinically detectable pharmacologic effects from F-18-PI-2620 among the 22 subjects.
With these results that show F-18-PI-2620 targets brain regions known to have tau deposits in patients with Alzheimer's disease, Mueller and colleagues endorsed additional research with "larger numbers of subjects and involving other tauopathies ... to further characterize and validate this tracer.
Disclosure note: Life Molecular Imaging provided financial support for the study.