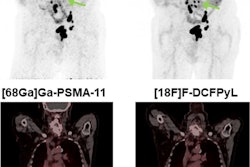
Hoag healthcare system in Orange County, CA, has launched a clinical trial to assess the prostate cancer PET imaging radiotracer Pylarify in patients newly diagnosed with intermediate disease.
Currently, the radiotracer is approved in the U.S. for diagnosed prostate cancer patients to identify suspected metastasis or recurrence of the disease. The trial will use Pylarify with whole-body PET/CT scans in men with favorable intermediate prostate cancer, including Gleason Score 3+4 tumors, Hoag said.
The phase IV open-label multicenter trial will ultimately assess the safety and accuracy of the approach in an estimated 274 participants, Hoag said.
“The MIRROR trial aims to bridge the gap in prostate cancer management by evaluating the utility of Pylarify imaging in favorable intermediate prostate cancer," noted study lead Gary A. Ulaner, MD, PhD, of the Hoag Family Cancer Institute, in a news release.