Theranostics is increasingly being used to image and treat prostate cancer and neuroendocrine tumors. Women's theranostics appears to already be on the horizon.
Several presenters at the Society for Nuclear Medicine and Molecular Imaging's (SNMMI) 2024 meeting in Toronto shared new findings in women's studies.
Theranostics is increasingly being used to image and treat prostate cancer and neuroendocrine tumors. Women's theranostics appears to already be on the horizon.
Several presenters at the Society for Nuclear Medicine and Molecular Imaging's (SNMMI) 2024 meeting in Toronto shared new findings in women's studies.
CAM-HER2 therapy
Belgium-based Precirix, for example, highlighted CAM-H2 therapy, a human epidermal growth factor receptor 2-directed ligand (HER2) camelid single-domain antibody (sdAb) labeled with radioisotope iodine 131 (I-131).
Called CAM-H2, the targeted radioligand therapy is intended for HER2-positive lesions in the treatment of metastatic HER2-positive cancer indications. Precirix recently completed a study of CAM-H2 in breast cancer, gastric cancer, and gastroesophageal junction cancer.
Preliminary data showed that CAM-H2 demonstrated high affinity for the HER2 target and rapid tissue penetration, explained Josie Gayton, PhD, during her presentation at SNMMI. Gayton oversees clinical operations at Precirix and serves as the company's chief development officer.
"HER2 is still a very major health burden despite the advantages in treatment options over the past few years," Gayton said during her talk. "With CAM-H2, we engineered the protein to bind to a different epitope on HER2 to other approved therapies, so the option of combining CAM-H2 with other therapies is definitely an opportunity."
The CAM-H2 dose escalation, safety, and dosimetry study in 13 patients, mostly women ages 50 to 63, occurred at four sites in Canada and the U.S. and included limited single and cumulative radiation doses. Patients included were HER2-positive (based on previous history) and had been heavily pretreated with systemic therapy and external beam radiotherapy. Nine had breast cancer, four of whom had brain metastases. Two had gastric cancer and two had gastroesophageal junction cancer.
Precirix's study examined CAM-H2-1-131 administration at three dose levels. Patients received up to four doses of CAM-H2 at 1.85 GBq (50 mCi), 3.7 GBq (100 mCi), and 5.55 GBq (150 mCi) dose levels given every four weeks.
"If we think about the dose levels we looked at, the first two cohorts were very low, subtherapeutic," Gayton said. SPECT/CT scans over time showed high specificity of CAM-H2 in reaching HER2+ lesions and rapid uptake of five hours with "good retention over time," especially in a case of HER2-positive brain metastases still lighting up at 168 hours (about seven days), Gayton said.
Researchers found the overall safety profile acceptable at the administered doses, according to Gayton. In addition, the mean tissue dose did not reach the maximum tolerated dose for kidney, bone marrow, or liver. Three grade 3 CAM-H2 treatment emergent adverse events (TEAE) were reported in three patients who had a previous history of thrombocytopenia, lung disease, or liver disease, with no grade 4 or 5 adverse events reported.
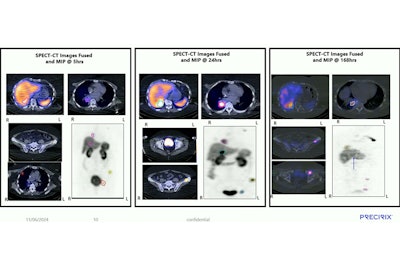 CAM-H2-I-131 is specific, reaches HER2 positive lesions, and is retained in lesions for at least seven days, according to research presented by Precirix during SNMMI 2024.Josie Gayton, PhD, Precirix
CAM-H2-I-131 is specific, reaches HER2 positive lesions, and is retained in lesions for at least seven days, according to research presented by Precirix during SNMMI 2024.Josie Gayton, PhD, Precirix
Gayton noted, however, that the study offered limited evidence of efficacy; the best result observed in three patients was confirmed stable disease.
CAM-H2 strategies will be evaluated next for phase II trials. "The results of this study support a proof of mechanism for the single-domain antibody-radioligand platform and will be instrumental in the planning of a phase II study of CAM-H2 for treatment of HER2-positive brain metastases," Gayton said.
NeoB PET imaging in breast cancer
Continuing along the HER2 line in women's theranostics, patients with estrogen receptive positive (ER+) and HER2-negative (HER2-) breast cancer showed the highest rate of NeoB-positive disease (90% positive in any lesion) in the first results of a prospective observational trial of 29 breast cancer patients, according to Dr. David Kersting of the Universitatsmedizin Essen in Germany.
NeoB can be used to identify candidates with sufficient tumor uptake for Lu-177-NeoB radionuclide therapy. Kersting's SNMMI 2024 presentation focused on PET imaging using gallium 68 (Ga-68)-NeoB, a novel DOTA-coupled gastrin releasing peptide receptor (GRPR)-targeting antagonist (previously called Ga-68-NeoBOMB1), in breast cancer patients.
GRPR is highly expressed in breast cancer as was observed in 75.8% of 1,432 tissue samples, and in lymph node metastases (94.6%) where the primary tumor was GRPR-positive, according to Kersting.
The study examined tumor uptake of Ga-68-NeoB on 26 scans in 29 patients: 10 from patients with newly diagnosed breast cancer, 16 from patients with persistent or recurrent disease, and three from patients without clinical verification of an active tumor.
Twenty of the 26 scans from patients with active tumors were NeoB-positive, six NeoB-negative. A region-based analysis -- i.e., breast, lymph node, liver, bone, and other distant metastases -- found a variety of tumor uptake values, Kersting said. Lymph node and distant metastases showed a tendency toward higher uptake than breast lesions. Eighteen of the 20 were ER+/HER2-.
Thus, ER+/HER2- breast cancer showed higher rates of uptake (90% in patient-based analysis) than patients with other hormone receptor status, Kersting noted. Global uptake was larger than liver uptake (defined as mean peak tumor-to-liver ratio from all NeoB-positive regions per patient being larger than 1) in about two-thirds of ER+/HER2- patients.
These findings were consistent with pathology data previously published by other groups, Kersting noted, adding that NeoB is a "highly interesting target" in combination with Lu-177 (Lu-177-NeoB) as a theranostic option.
Separately, Novartis has a first-in-human study of Lu-177-NeoB underway to characterize safety, tolerability, pharmacokinetics, distribution, radiation dosimetry, and antitumor activity in patients with advanced solid tumors known to overexpress GRPR and with Ga-68-NeoB lesion uptake.
FES PET/CT for initial staging of breast cancer
"There are multiple HER2-targeted PET radiotracers currently in clinical trials for the detection of HER2-positive malignancy, determination of disease extent, and selection of patients for HER2-targeted therapy," wrote Gary Ulaner, MD, PhD, director of molecular imaging and therapy at Hoag Family Cancer Institute, for a review and commentary published June 18 in Radiology.
Ulaner is a professor of radiology and translational genomics. He also serves as principal investigator of grants from the National Institutes of Health R01, U.S. Department of Defense, and foundations for HER2-, ER-, PSMA-, and CD38-targeted PET radiotracers, as well as consulting and advising on several commercial scientific advisory boards.
Breast cancer metastases are often suboptimally detected by current standard of care imaging, which limits optimal treatment selection, Ulaner said at SNMMI.
While the fluoroestradiol F-18 radiotracer is approved by the U.S. Food and Drug Administration (FDA), it has appropriate use criteria defined for it which are more for helping to evaluate patients that will be hormone-responsive rather than for staging and for evaluation of disease recurrence, Ulaner explained.
"So the value of FES-PET in initial staging of patients with locally advanced breast cancer and in the detection of local recurrence is currently unknown," he said.
Thus, a phase II clinical trial was designed for 124 patients with ER+ breast cancer; 62 were locally advanced newly diagnosed cases, whereas the other 62 were suspected of recurrence based on signs symptoms or a laboratory value. Both groups would undergo standard of care imaging, or CT and bone scan, or FDG PET/CT, or FES PET/CT.
"Looking at the statistics in both arms of the study, the FES PET scan was statistically equivalent to the standard of care arm," Ulaner explained during his SNMMI talk. In the newly diagnosed cohort 1, standard of care imaging detected 12/14; FES detected 11/14 (P > 0.99). In the suspected recurrence cohort 2, standard of care imaging detected 16/23; FES detected 18/23 (P = 0.77).
"This supports the use of FES-PET for patients with estrogen receptor-positive breast cancer at both initial staging of locally advanced disease and in patients with suspected recurrence, as FES-PET performed equivalent to our current standards of care." Moreover, in the patients with lobular breast cancer, FES detected nearly twice as many sites of distant metastases or recurrences, Ulaner added.
Ulaner called the trend encouraging. "This is exciting and represents two novel patient populations that may benefit from FES-PET/CT, which is not considered in these patient populations," he said. "Maybe more importantly, there was a trend for superiority among patients with lobular breast malignancies."
"This should raise interest in the use of FES PET/CT for both of these clinical indications," Ulaner told AuntMinnie.com via email. Look for full details on Ulaner's study of ER-targeted PET for initial staging and suspected recurrence in ER-positive breast cancer at JAMA Open on or around July 26.
Cervical cancer-directed PET
Switching to cervical cancer, research in SNMMI's highlighted country South Africa is investigating chemokine receptor CXCR4-directed PET imaging for earlier cervical cancer detection and selecting potentially more effective treatments.
Cervical cancer is the leading cause of death for women and girls from ages 15 to 44 in low and middle-income countries such as South Africa, according to SNMMI presenter Bawinile Hadebe, MD, whose team from the University of KwaZulu-Natal sought to establish the value of CXCR4-directed PET imaging in patients with cervical carcinoma using the radiotracer Ga-68 PentixaFor, compared to F-18 FDG.
The small study involved 15 women around 46 years old with squamous cell carcinoma and moderate tumor involvement. Preliminary results showed lower uptake with Ga-68 PentixaFor (detecting 37 lesions) compared to F-18 FDG (detecting 68 lesions), according to Hadebe.
"We need to find solutions for cervical cancer in our environment, especially since our patients present late and when they present they often already have advanced disease and metastatic disease which is challenging to treat," Hadebe said.
In another study out of South Africa, University of Pretoria researchers compared the Ga-68 fibroblast activation protein inhibitor (FAPI) PET/CT with F-18 FDG PET/CT in imaging cancer of the cervix. The study involved 19 patients and found Ga-68 FAPI PET detected more visceral and skeletal metastases (9 vs 4) and (10 vs 5) compared to F-18 FDG PET/CT. Researchers also determined Ga-68 FAPI PET has potential for pairing with Lu-177 FAPI in theranostics, according to Kgomotso Mokoala, MD, who presented on behalf of the work.
This article is Part 4 of the series: The rise of theranostics.

