Researchers may be a step closer to using "regenerative imaging" to monitor in vivo cell dynamics after stem cell therapy for the treatment of neurological disorders, according to a proof-of-concept study published September 2 in Radiology.
The findings provide a deeper understanding of stem cell dynamics in ischemic stroke and suggest a system for long-term, multimodal monitoring of cell-based therapies, specifically human neural progenitor cell (hNPC) transplantation, according to Xiaoyi Li, MD, PhD, of the Hangzhou Institute of Medicine and Yan Zhong, MD, PhD, of the Zhejiang University School of Medicine in Hangzhou, China.
"There is an urgent need for effective tools to monitor cell-based interventions," Li, Zhong, and colleagues wrote. "Neural progenitor cell therapy holds great potential for repairing brain damage induced by ischemic stroke, and molecular imaging plays a crucial role in evaluating the therapeutic efficacy of neural progenitor cell transplantation. However, the presence of the blood-brain barrier significantly limits the effectiveness of such imaging methods."
A multidisciplinary team made up of nuclear medicine, molecular imaging, and biomedical engineering researchers created a platform that combines a clustered regularly interspaced short palindromic repeats (CRISPR)-associated nuclease-9 (Cas9)-engineered triple-fusion (TF) reporter gene imaging system with a noninvasive agonistic micelle (AM)-based molecular probe delivery approach.
The reporter gene imaging system incorporated the sequence of HSV1-tk to enhance detection sensitivity on PET, Fluc for bioluminescence imaging, and GFP for fluorescence imaging to enable longitudinal tracking of transplanted hNPCs in rat brains, according to the group.
Using a rat model of ischemic stroke, researchers integrated the reporter gene system into human embryonic stem cells (hESCs), which were differentiated into TF-hNPCs. Transplanted rats were randomly divided into two groups and treated with phosphate-buffered saline or AMs, respectively. Following overnight fasting, the rats underwent F-18 fluorodeoxyglucose PET imaging.
In vivo PET and bioluminescence imaging showed that TF-hNPCs proliferated (week 8 vs. week 1 for bioluminescence and PET imaging: p < 0.001 and p = 0.02, respectively), differentiated (proportion of microtubule-associated protein 2-positive TF- hNPCs at week 8 vs. week 4, 94.08% ± 3.02 vs. 85.47% ± 6.54, respectively; p = 0.04), and migrated in the ischemic brain, the group noted.
Also, TF-hNPC transplantation increased F-18 fluorodeoxyglucose uptake in the ischemic area from week 4 to 8 (p = 0.008 and p = 0.01 for TF-hNPCs vs. vehicle, for week 4 and week 8, respectively) and attenuated neurologic deficits (TF-hNPCs vs. vehicle at week 8, p = 0.003).
The researchers observed relatively low radioactivity in normal brain tissue, as seen on F-18-FHBG scans before and after AM injection. Notably, AMs increased the transplant-to-background ratio threefold, they said.
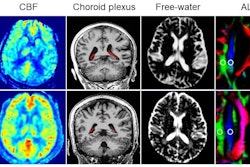
 Representative images and plots show neurologic and cerebral glucose metabolic changes after triple-fusion (TF) human neural progenitor cell (hNPC) transplantation in a rat stroke model. (A) Representative cerebral F-18 fluorodeoxyglucose PET images show the metabolic changes in TF-hNPCs or vehicle-treated rats with middle cerebral artery occlusion at day 1 after middle cerebral artery occlusion (before vehicle injection or TF-hNPC transplantation) and at weeks 1, 2, 4, and 8 after transplantation. Images show that TF-hNPC therapy attenuated cerebral glucose metabolic damage in the ischemic brain region (arrows). The color bar shows the PET signal intensity ... blue shows low intensity, whereas yellow and red indicate higher intensity. (B) Scatterplot of semiquantitative analysis of cerebral glucose metabolism ... (C) Scatterplot of Garcia neurologic scores ... (B, C) Data are presented as means with individual replicates. L/N = lesion-to-normal ratio, p.t. = posttransplantation.Image and caption courtesy of RSNA.
Representative images and plots show neurologic and cerebral glucose metabolic changes after triple-fusion (TF) human neural progenitor cell (hNPC) transplantation in a rat stroke model. (A) Representative cerebral F-18 fluorodeoxyglucose PET images show the metabolic changes in TF-hNPCs or vehicle-treated rats with middle cerebral artery occlusion at day 1 after middle cerebral artery occlusion (before vehicle injection or TF-hNPC transplantation) and at weeks 1, 2, 4, and 8 after transplantation. Images show that TF-hNPC therapy attenuated cerebral glucose metabolic damage in the ischemic brain region (arrows). The color bar shows the PET signal intensity ... blue shows low intensity, whereas yellow and red indicate higher intensity. (B) Scatterplot of semiquantitative analysis of cerebral glucose metabolism ... (C) Scatterplot of Garcia neurologic scores ... (B, C) Data are presented as means with individual replicates. L/N = lesion-to-normal ratio, p.t. = posttransplantation.Image and caption courtesy of RSNA.
Ultimately, the platform improved blood-brain barrier (BBB) permeability without inducing brain edema, hemorrhage, or other adverse effects, according to the study authors.
While the long-term fate of the cells and the sustained functional improvement beyond eight weeks were not determined, the study is considered a milestone. In an accompanying editorial, Fanny Chapelin, PhD, from the departments of bioengineering and radiology at the University of California, San Diego, said the work provides a foundation for advancing stem cell imaging.
"Without a way to circumvent the BBB, tracking cells in the [central nervous system] CNS using PET would be very limited," Chapelin wrote. "Biologically, this work creates chances for systemic studies of cell therapy mechanisms. Li and Zhong et al present a relevant and thorough study that advances the field of regenerative imaging."
From a clinical viewpoint, the approach presented here may be especially useful for preclinical safety and effectiveness studies, Chapelin added.
Read the complete paper here.