A novel PET radiotracer that targets vascular adhesion protein-1 (VAP-1) on blood vessel walls shows promise for imaging pulmonary sarcoidosis, researchers have reported.
The finding is from a proof-of-concept trial in a small number of patients, yet suggests the technique may overcome limitations of current molecular imaging approaches, noted lead author Prince Dadson, MD, PhD, of the University of Turku in Finland, and colleagues.
“Although F-18 FDG-PET/CT is widely used to evaluate sarcoidosis and other leukocyte-mediated disorders, its clinical utility is limited by nonspecific uptake in any metabolically active tissue. This hampers differentiation between inflammatory and malignant lesions,” the group wrote. The study was published December 19 in Respiratory Research.
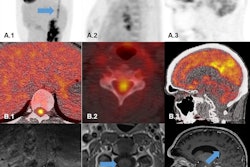
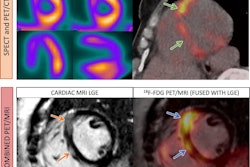
Sarcoidosis is a chronic inflammatory disease that causes granulomas to form in organs, most commonly in the lungs, the researchers explained. To meet the need for more specific molecular imaging tools that target the disease, the group developed gallium-68 (Ga-68) DOTA-Siglec-9, a PET radiotracer that binds to VAP-1. VAP-1 is a protein expressed by vascular endothelial cells in blood vessels during inflammation. A graphical abstract of the study.Respiratory Research
A graphical abstract of the study.Respiratory Research
In this study, the researchers evaluated Ga-68 DOTA-Siglec-9 PET/CT for the first time in six patients (average age, 50.5 years old) with stage II pulmonary sarcoidosis and compared findings to those in six healthy male volunteers. They analyzed radiotracer uptake in the lungs using standardized uptake values (SUV).
According to the results, patients with sarcoidosis showed significantly higher Ga-68 DOTA-Siglec-9 uptake in the lungs (SUVmean 1.82 vs. 0.41; p = 0.00006) and in mediastinal lymph nodes (SUVmean 2.06 vs. 0.89; p = 0.0003) compared with healthy controls.
In addition, increased uptake was also observed in the liver (SUVmean 1.18 vs. 0.8; p = 0.0003), spleen (SUVmean 1.13 vs. 0.82; p = 0.00003), bone marrow (SUVmean 0.3 vs. 0.06; p = 0.001), and bone (SUVmean 0.27 vs. 0.08; p = 0.004), indicating systemic inflammation.
“Our findings suggest this approach may enhance diagnostic accuracy and provide a more targeted method for monitoring disease activity,” the group wrote.
Ultimately, this was a proof-of-concept study and the findings must be considered preliminary, the researchers noted. They also highlighted that the study participants lacked ethnic diversity and that future research should include more diverse populations, given that Black patients with sarcoidosis are more likely to experience severe pulmonary involvement.
Importantly, however, the results of this study contribute to the growing understanding VAP-1’s role in sarcoidosis pathophysiology, they wrote.
“Further validation in larger cohorts are warranted,” the group concluded.
The full study is available here.