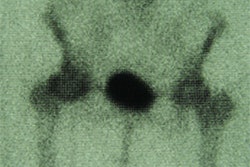
Researchers at the University of California, San Diego Cancer Center have filed an application with the Food and Drug Administration to begin investigational clinical evaluation of a radiolabeled agent. The agent is designed to assist in the surgical management and diagnosis of breast cancer patients, according to its manufacturer, gamma-guided surgery firm Neoprobe.
A radioactive isotope is attached to the agent, which has shown the ability in preclinical studies to accurately identify lymphatic tissue, Dublin, OH-based Neoprobe said. The phase-I clinical study at UCSD will evaluate the agent’s ability to accurately identify tumor-draining lymph nodes when used with the firm's handheld gamma radiation detection instruments.
Neoprobe has completed an option agreement with the regents of the University of California to exclusively license the proprietary UCSD agent.
By AuntMinnie.com staff writersFebruary 14, 2001
Copyright © 2001 AuntMinnie.com