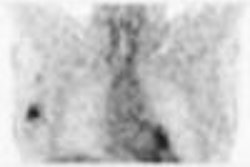
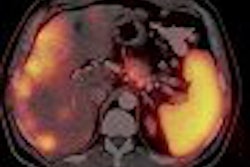
Canadian radioisotope developer MDS Nordion reported that it has received U.S. Food and Drug Administration (FDA) approval to begin clinical trials of its TheraSphere treatment for liver cancer.
The TheraSphere treatment consists of yttrium-90 filled glass microspheres that are injected into the main artery of a patient's liver via catheter, the company said.
The clinical trial, expected to begin in October, will enroll up to 150 patients with secondary liver cancer from five existing TheraSphere treatment centers in the U.S., according to the Kanata, Ontario-based firm.
By AuntMinnie.com staff writers
September 26, 2006
Related Reading
MDS Nordion Q3 posts flat revenues, September 7, 2006
MDS Nordion Q2 shows gains, June 8, 2006
MDS Nordion moves closer to end users, June 7, 2006
MDS Nordion, Molecular Insight Pharmaceuticals partner, February 16, 2006
MDS Nordion collaborates with GSK, November 27, 2005
Copyright © 2006 AuntMinnie.com