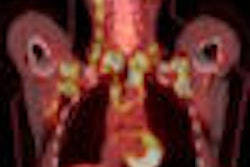
Cardiac molecular imaging company Positron has received U.S. Food and Drug Administration (FDA) approval for a new PET scanner product line, the Fishers, IN-based company said.
Neusoft Positron Medical Systems, Positron's joint venture partner based in Shenyang, China, got the FDA nod for Positron's Attrius PET scanner. The system includes a coronary artery disease software suite that features Mercator projections, normal database comparisons, coronary artery tree and distribution overlay maps, rapid segmented attenuation correction, motion correction, and open acquisition architecture for quantitative flow reserve imaging.
Related Reading
Positron places bets on PET-only Attrius system, March 4, 2009
Neusoft Positron files 510(k) for Attrius, January 29, 2009
Positron launches cardiac nuclear medicine package, January 9, 2009
Positron hires medical director, December 17, 2008
Neusoft taps TUV for FDA help, October 23, 2007
Copyright © 2009 AuntMinnie.com