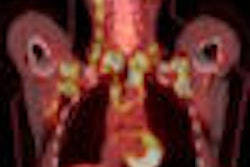
PhotoDetection Systems (PDS) has received approval from the U.S. Food and Drug Administration (FDA) for its NeuroPET scanner, the Boxboro, MA-based company said.
The device features wavelength-shifting fiber technology that allows for high sensitivity and spatial resolution. Its compact design requires no site preparation and reduces the cost of ownership compared to general-purpose PET scanners.
The system also uses low doses of fluorine-18 FDG and C-11 choline tracers to image metabolism, amyloid, and dopaminergic binding in neurological diseases, according to PDS.
Related Reading
PDS pushes PET's imaging boundaries with lower doses, January 22, 2009
Analogic acquires minority stake in PhotoDetection Systems, May 23, 2003
Copyright © 2009 AuntMinnie.com