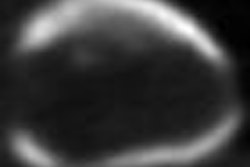
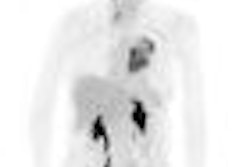
Molecular imaging developer Avid Radiopharmaceuticals said that two presentations of phase II trial results at this week's 2009 International Conference on Alzheimer's Disease in Vienna, Austria, support the efficacy of its fluorine-18 (F-18) AV-45 PET agent for molecular imaging of amyloid aggregates in the brain.
One of the main conclusions of the phase II trial is that elevated brain amyloid levels in cognitively normal subjects, as measured by F-18 AV-45 PET imaging, are associated with decreased memory performance, according to Philadelphia-based Avid.
The study also found that the mean F-18 AV-45 retention values in the brain differed significantly between healthy control (HC) and Alzheimer's disease (AD) populations (p < 0.001). In addition, the results showed that mild cognitive (MCI) brain amyloid levels, on average, fall in between HC and AD subjects, with individual patient results reflecting one extreme (e.g., high, AD-like amyloid levels) or the other (e.g., low, HC-like amyloid levels), Avid said.
Dr. Reisa Sperling from Massachusetts General Hospital in Boston and Dr. P. Murali Doraiswamy of Duke University Medical Center in Durham, NC, presented the study results.
Related Reading
Avid closes on financing, May 22, 2009
Michael J. Fox Foundation awards neuroimaging grants, February 19, 2009
Avid launches clinical trial, October 3, 2007
Bayer Schering Pharma licenses Avid's imaging agent, June 19, 2007
UPenn study awarded SNM Small Animal Image of the Year, June 15, 2007
Copyright © 2009 AuntMinnie.com