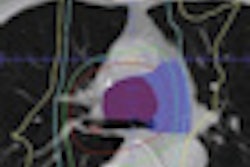
Radiopharmaceutical developer Cell>Point of Centennial, CO, is moving toward a phase III clinical trial for its SPECT imaging agent after encouraging preliminary results showed the agent's ability to match FDG-PET in diagnosing and staging patients with cancer.
Results from the company's six-site phase II clinical trial, which ended in July, suggest that technetium-99m (Tc-99m) ethylene dicysteine deoxyglucose (ECDG) with SPECT can localizable primary and metastatic lesions and is not inferior to FDG-PET in image quality or performance.
"In our preliminary analysis, all the primary and all the metastatic lesions we see on FDG-PET we also see on the ECDG-SPECT," said Cell>Point President F. David Rollo, MD, PhD.
In addition, ECDG-SPECT allowed researchers to see an uptake of the contrast agent in metastasized sites on long bones, such as an arm or leg, which could not be seen with FDG-PET. "The reason we don't see it on PET is that FDG localizes in bone marrow" and not on bones, Rollo explained.
He cited one case in which researchers found a tumor on a patient's sternum. "When you look at the image, you can see that the tumor probably started in the bone marrow and eroded into the bone, and we get uptake in both our agent and the FDG," he added.
Phase III trial
If and when Cell>Point proceeds to a phase III trial, it plans to further investigate this finding and determine if ECDG-SPECT can consistently detect both primary and metastatic lesions and metastatic lesions that include the bone.
The next immediate step this month is to conduct additional image analysis of phase II results with help from Biomedical Systems in St. Louis. Expert readers will interpret the images in a blinded protocol. One group will evaluate the FDG-PET images, while a second group will review the ECDG-SPECT images.
Cell>Point then plans to present the results to the U.S. Food and Drug Administration (FDA). "The plan is to have a face-to-face meeting with the FDA to discuss the clinical results, and to discuss with them our proposal for phase III [clinical testing] in terms of the claims we would like to make when the study is completed, and to make sure the agency agrees that our protocol is adequate to support the claims," Rollo said.
The company anticipates sending its report to the FDA by mid-August with a letter to the agency to request the follow-up meeting in late August or early September.
"Once we have had that meeting and they give us instructions in terms of what they believe we need to do to complete the study, then hopefully [the phase III clinical trial] will begin in the fall," Rollo added.
Phase I results
In June, Rollo presented phase I clinical trial results, which showed ECDG-SPECT's equivalency to FDG-PET/CT for diagnosing and staging non-small cell lung cancer (NSCLC), at the annual meeting of SNM in Salt Lake City.
In that study, researchers from Cell>Point and M. D. Anderson Cancer Center at the University of Texas in Houston compared the efficacy of the two imaging techniques in seven patients with confirmed cases of NSCLC who had received chemotherapy.
(Initially, the preference was to enroll patients who had not received any chemotherapy, but after 18 months or so, the researchers did not have a single patient for the study. "It was just impossible to recruit people because once they found out they had cancer, they wanted to be treated right away," Rollo said. The protocol was changed to patients with a confirmed diagnosis of non-small cell lung cancer and patients who were already in therapy.)
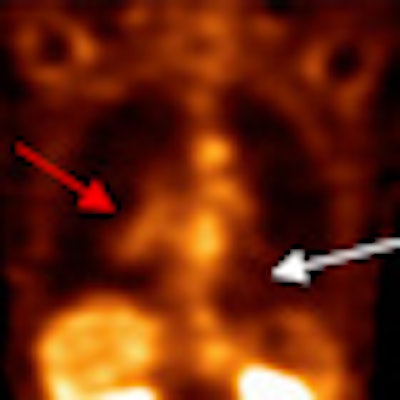
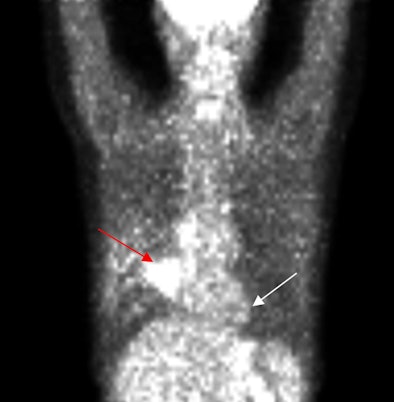 |
| PET scan, above, indicates the presence of a large tumor (red arrow); the corresponding ECDG-SPECT image, below, also defines the presence of the mass (red arrow). The FDG-PET image also shows benign pulmonary nodules (white arrow), which are not see on the ECDG-SPECT image, indicating they are not representative of cancer. Images courtesy of F. David Rollo, MD, PhD, and Cell>Point. |
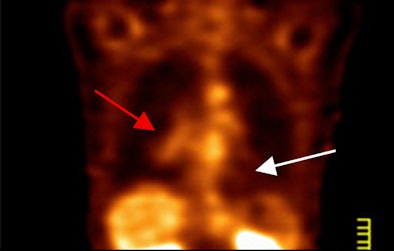 |
The study found that ECDG-SPECT matched FDG-PET in image interpretation for all primary lesions. With FDG-PET, the researchers wrote, primary lesions had a "fluffy appearance and were larger than noted on pretreatment PET, consistent with chemotherapy inflammatory response. For SPECT, the primary lesions were smaller and more discrete, consistent with clinical findings of chemotherapy efficacy."
Glucosamine contribution
Rollo said the key element of ECDG is glucosamine, which is involved in cell replication, as opposed to glucose, which is used for cell energy and respiration. "The function of the agent was to get involved in any cells that are undergoing rapid replication, and that, of course, includes oncology, as opposed to FDG, which is involved in anything that requires energy consumption," he added.
Hence, preclinical work on ECDG revealed that the agent localized in every type of cancer, including lung cancer, prostate cancer, colon cancer, and head and neck cancer. "It gave credence to the theory that the glucosamine is involved in any cell that is undergoing rapid replication," Rollo said. "Therefore, it is logical that it would be localized in various forms of cancer."
Cell>Point's IND
Cell>Point also is exploring uses for another of its contrast agents. The company has submitted an investigational new drug (IND) application to the FDA for a radiopharmaceutical for imaging coronary artery disease.
The IND covers a phase Ib/II trial of the company's technetium-99m-labeled glucosamine (Tc-99m EC-G) diagnostic imaging agent. It is designed to evaluate patients with cardiovascular issues, such as reduced blood supply (ischemia), recent heart attacks, and questionable heart muscle cell viability.
The study will attempt to determine if Tc-99m EC-G can be used as an alternative to traditional myocardial perfusion imaging (MPI) agents and be target-specific for the region of ischemia.
Preclinical studies have shown that the agent targets and concentrates in ischemic heart muscle tissue.
By Wayne Forrest
AuntMinnie.com staff writer
August 20, 2010
Related Reading
Cell>Point submits IND for heart agent, June 23, 2010
Cell>Point touts SPECT agent results at SNM, June 9, 2010
Cell>Point names Rollo as president, September 13, 2006
Biotech firm Cell>Point sees promise for oncology SPECT, May 20, 2005
Philips adds molecular imaging partnership, September 29, 2003
Copyright © 2010 AuntMinnie.com