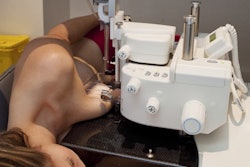
Breast biopsy developer Mammotome has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Mammotome Molecular Imaging (MI) system.
Mammotome MI can be used to conduct vacuum-assisted breast biopsy under guidance from molecular breast imaging modalities, and it's commercially available to healthcare facilities specializing in breast cancer screening and diagnosis, according to the Cincinnati-based division of Devicor Medical Products.
In other developments, Mammotome said its new corporate owners have committed to investing more than $60 million in R&D over the next two years in hopes of quickly adding new offerings to the Mammotome product portfolio. Devicor acquired the technology in its purchase of Ethicon Endo-Surgery's breast care business from Johnson & Johnson in July.
Mammotome also plans to establish a new manufacturing facility and add more jobs over the next six to nine months. The firm has more than 300 employees around the globe and plans to add more than 150 jobs over the next year.
Related Reading
Ethicon to sell biopsy unit, March 30, 2010
Copyright © 2010 AuntMinnie.com