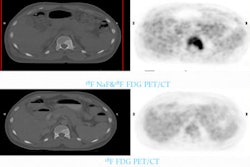
Antibody-directed drug and radionuclide therapeutics for pancreatic cancer improved efficacy with minimal toxicity in preclinical studies, according to results presented at the Gastrointestinal Cancers Symposium held last week in San Francisco.
Research conducted by R.M. Sharkey, PhD, director of clinical research administration at the Garden State Cancer Center in Belleville, NJ, and colleagues demonstrated the feasibility of using antibody-drug conjugates for treating pancreatic cancer, according to biopharmaceutical firm Immunomedics of Morris Plains, NJ.
The study of mice bearing human pancreatic cells used two of the company's proprietary humanized antibodies. When antibody-drug conjugates were combined with a radiolabeled antibody, time to progression improved considerably and more mice became free of tumors.
The combination of antibody-drug conjugates and radiolabeled antibody therapies was best when the latter was administered one to two weeks before, the same day, or within one week of the antibody-drug conjugates treatment, according to Sharkey in his poster presentation. Delaying radioimmunotherapy for two weeks after the antibody-drug conjugates treatment reduced efficacy.
Overall response was further enhanced when gemcitabine was added to the combination therapies, according to the researchers.
Related Reading
Immunomedics adds to patent claims, October 12, 2010
New promise for therapeutic radiopharmaceuticals, June 6, 2006
Immunomedics to appeal Nasdaq ruling, June 15, 2005
Immunomedics loss widens, February 7, 2005
Immunomedics gets Canadian nod for LeukoScan, January 19, 2005
Copyright © 2011 AuntMinnie.com