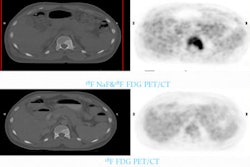
Nuclear medicine firm Areva Med of Bethesda, MD, has received approval from the U.S. Food and Drug Administration (FDA) to begin U.S. clinical trials of its lead-212 radioactive isotope as a new treatment for cancer.
The radioisotope uses a technology known as alpha radioimmunotherapy to pinpoint and destroy cancer cells, while limiting toxicity to healthy cells. Areva developed the process for extracting lead-212 from thorium derived from its industrial activities.
Phase I clinical trials are scheduled to begin this year in the U.S. and will take approximately two years to complete.
Related Reading
Schering buys rest of CIS bio, January 3, 2002
Copyright © 2011 AuntMinnie.com