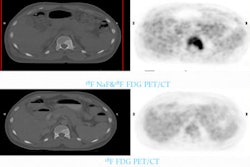
The U.S. Food and Drug Administration (FDA) has approved an investigational new drug application (IND) for an ultrasmall silica inorganic nanoparticle platform for targeted molecular imaging of cancer.
The application was submitted by researchers at Memorial Sloan-Kettering Cancer Center's Nanotechnology Center in New York City; Cornell University in Ithaca, NY; and Hybrid Silica Technologies of Cambridge, MA.
Researchers plan to launch their first human clinical trial in melanoma patients using the nanoparticle. The goal of the trial is to evaluate the distribution, tissue, uptake, and safety of the particles in humans by PET imaging. This study will provide data that will serve as a baseline to guide the design of future surgical and oncologic applications in the clinic.
The technology is based on Cornell dots (C dots), which were initially developed as optical probes by Ulrich Wiesner, PhD, a professor of materials science and engineering at Cornell, with C dots supplier Hybrid Silica Technologies. C dots were subsequently modified at Memorial Sloan-Kettering for use in PET imaging.
Related Reading
GE, NCI to research nanoparticle-based imaging agents, September 22, 2008
Stanford researchers on path to novel molecular imaging technique, April 10, 2008
UM researchers develop 'green' nanoparticles for cancer treatment, December 7, 2007
Copyright © 2011 AuntMinnie.com