FDG-PET is sensitive and specific in detecting bone marrow involvement in pediatric Hodgkin's lymphoma patients and may be a safe alternative to an invasive bone marrow biopsy in routine staging procedures, according to a study published online August 8 in the Journal of Clinical Oncology.
Researchers from the University of Leipzig and Charité Medical University in Germany concluded that FDG-PET's high sensitivity and specificity could eliminate the need for a routine bone marrow biopsy. Skeletal PET findings could also potentially change the regimen for pediatric Hodgkin's lymphoma patients to more appropriate and effective treatment.
Currently, bone marrow biopsy is the only invasive staging procedure to detect bone marrow involvement in Hodgkin's lymphoma, according to lead study author Dr. Sandra Purz, from the University of Leipzig, and colleagues. Detecting bone marrow involvement in these cases is particularly important because it can confirm stage IV disease and the need for more intense treatment.
A total of 676 children newly diagnosed with Hodgkin's lymphoma were enrolled in the multicenter study between 2002 and 2006. Initial disease staging included chest CT scans; MR or CT scans of the neck, abdomen, and pelvis; and bone marrow biopsies in patients with Hodgkin's lymphoma greater than stage IIA. Children who were suspected to have skeletal involvement were recommended for bone scintigraphy (Journal of Clinical Oncology, August 8, 2011).
Imaging protocols
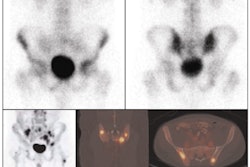
Dedicated PET or PET/CT systems (Biograph Duo, Ecat Exact HR+, Siemens Healthcare; Allegro, Philips Healthcare; Discovery LS, GE Healthcare) were used to scan patients after a fasting period of four to six hours. Image acquisition began 40 to 90 minutes after the intravenous injection of FDG based on the child's body weight.
In addition, CT images were acquired of all regions from the head to the lower edge of the pelvis, using oral contrast and IV contrast. MRI scans of the neck, abdomen, and pelvis included T2-weighted, fat-saturated, transverse and coronal sequences and T1-weighted dynamic sequences with a contrast agent.
Only patients with pathologic skeletal findings indicated by bone marrow biopsy or PET, CT, or MRI scans were recommended for whole-body bone scintigraphy. Bone marrow biopsies were performed in standard regions at the iliac crest.
A total of 404 of the 676 patients presented with Hodgkin's lymphoma greater than stage IIA. Staging FDG-PET scans were performed on 199 of the 404 patients before treatment began. Because no bone marrow biopsies were performed on 24 of the 199 patients, no results were available, and those patients were excluded from the study. The final study sample included 175 patients (89 males and 86 females) with a mean age of 14.6 years.
PET-positive results
The researchers found that 45 (26%) of the 175 patients with Hodgkin's lymphoma greater than stage IIA were PET-positive for bone marrow involvement.
In 32 of the 45 patients, three or more skeletal lesions were present on FDG-PET scans; 12 patients had only one such lesion, and one patient had two such lesions. Of these 13 patients, two patients had bone marrow biopsy-positive disease.
Only seven (4%) of the 175 patients had positive bone marrow biopsy results, with all seven having at least one FDG-PET-positive lesion in the skeleton.
Among 28 patients with a positive finding by MRI or CT, at least one lesion was FDG-PET positive. "Thus, no false-negative PET lesions were found," Purz and colleagues wrote. "Therefore, the sensitivity and negative predictive value of PET were 100% when using a combination of bone marrow biopsy, CT, or MRI results for reference."
In 25 of the 45 PET-positive patients, MRI results were available in at least one of the PET-positive skeletal areas and were positive in 23 (92%) of 25 FDG-PET-positive patients. Of the 45 PET-positive patients, 40 were evaluated for bone involvement by using bone scintigraphy (n = 36) and/or CT (n = 23). In 25 (62.5%) of the 40 patients, at least one of the PET-positive lesions showed a correlative finding on bone scintigraphy (23 of 36) and/or CT (10 of 23) scans.
For the 168 bone marrow biopsy-negative patients, FDG-PET results were negative in 127 cases and questionable in three patients.
Chemotherapy
In 38 FDG-PET-positive patients, follow-up PET scans after two, three, four, or six chemotherapy treatments showed that the majority of skeletal lesions detected by the modality had disappeared.
A comparison of event-free survival between the 130 PET-negative and 45 PET-positive patients suggested a trend toward worse prognoses among patients whose pathology was detected on PET scans. However, Purz and colleagues concluded that the results were not statistically significant because of the overall low rate of events.
The study results confirm that FDG-PET's high sensitivity and specificity could bypass the need for a routine bone marrow biopsy, according to the authors. "In place of a bone marrow biopsy, a typical multifocal pattern of skeletal PET findings could be considered another means of assessing bone marrow involvement in patients with Hodgkin's lymphoma," they wrote.
The group recommended a targeted biopsy in cases of solitary skeletal PET findings in which a diagnosis of bone marrow involvement would change the patient's treatment recommendation.