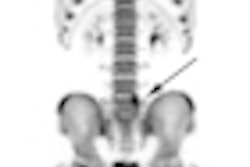
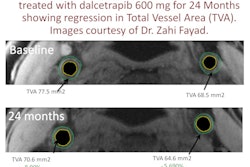
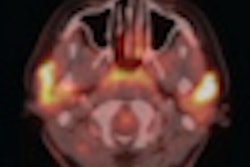
PET developer Naviscan has received the CE Mark for both its high-resolution positron emission mammography (PEM) scanner and PET-guided biopsy accessory.
The approval enables the company to market the system in Europe, where it has set up distribution in 13 countries, Naviscan said. The firm has already received the first two orders for the system in Germany and Switzerland.Naviscan's PEM gets CE Mark
Latest in Nuclear Medicine
Andrew Trout tapped to lead radiology at Cincinnati Children's
October 17, 2025
SpectronRx’s Ac-225 boosted for use in Canada
October 1, 2025
NAPT survey results show promise for proton therapy adoption
September 29, 2025