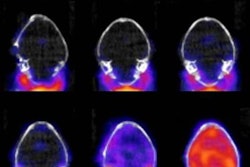
Molecular imaging firm Blue Earth Diagnostics is highlighting interim clinical results at the American Society for Radiation Oncology (ASTRO) meeting in San Diego from a trial involving its Axumin (fluciclovine F-18) PET radiopharmaceutical.
The FALCON trial -- Fluciclovine (F-18) PET/CT in Biochemical Recurrence of Prostate Cancer -- is a U.K.-based study evaluating the clinical impact of fluciclovine F-18 PET/CT imaging on patient management decisions in men with recurrent prostate cancer.
Interim results showed that 61.2% of patients had their clinical management changed when results of fluciclovine F-18 PET/CT imaging were added to the standard diagnostic workup, according to a team led by Dr. Eugene Teoh of Oxford University Hospitals NHS Foundation Trust. Major revisions were made for 59.6% of patients. Salvage treatment was revised to watchful waiting for 25% of patients and to systemic therapy for 34.6% of patients, while 40.4% of patients had their previously planned radiotherapy field modified, Blue Earth said.
The trial is funded by Innovate U.K. and Blue Earth Diagnostics and is being conducted at six institutions.