MRI contrast developer Epix Pharmaceuticals said that the Food and Drug Administration has accepted the firm's May response as a complete response to the FDA's approvable letter for its Vasovist (MS-325) MR blood pool contrast agent.
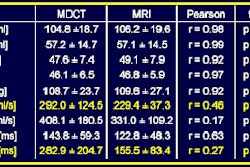
The FDA said it would review the new drug application (NDA) with a target date for an action letter of late November, according to the Cambridge, MA-based firm. The agency also encouraged Epix to consider a reread of the images from the phase III trials to provide additional information about the usefulness of dynamic versus steady-state images, and to schedule a meeting with the FDA to discuss the merits and design of such a study, Epix said.
Epix said it would schedule that meeting as soon as possible. The company said it also asked the FDA to review the NDA, including the complete response, in parallel with those discussions. The FDA has agreed to do so, Epix said.
By AuntMinnie.com staff writers
July 1, 2005
Related Reading
Epix submits response to FDA, May 23, 2005
Epix revenues, income down in Q1, April 29, 2005
Epix launches blood-clot agent trial, April 7, 2005
Epix signs DeWitt, April 5, 2005
Bracco dispute trims Epix revenues, February 18, 2005
Copyright © 2005 AuntMinnie.com