The American College of Radiology (ACR) has released an updated version of its Manual on Contrast Media.
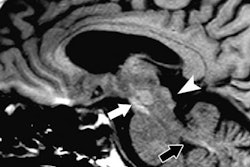
Version 10.2 contains a statement addressing the U.S. Food and Administration (FDA) 2015 safety communication on the risk of gadolinium-based MRI contrast agents leaving deposits in the brain, bone, and other organs.
The statement, jointly developed by the ACR and the American Society of Neuroradiology, describes how multiple factors need to be considered in deciding to use a gadolinium-based contrast agent (GBCA) for an MRI study. These include "diagnostic efficacy, relaxivity, rate of adverse reactions, dosing/concentration, and propensity to deposit in more sensitive organs, such as the brain," according to the organizations.
Other additions in the latest version of the manual include an updated footnote that covers the FDA's April 2016 guidance on patients who take the metformin medication (Glucophage) and receive contrast media. The ACR said it has also added a document version history and has incorporated information and new references about intraoseus contrast injection. The new manual can be downloaded here.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)