Brigham and Women's Hospital (BWH) in Boston is making a considerable investment in the future of radiology and patient care with its recent installation of an investigational 7-tesla MRI scanner. While the unit initially will be used for research, the hospital is open to the idea of someday using the scanner clinically.
Exploration into 7-tesla MRI and its benefits is still in the early stages; however, BWH is setting its sights on using the ultrahigh-field technology to advance its current and planned research projects. The early targets are the brain and the knee, where the magnet's strength creates a better signal, leading to higher resolution images of neurodegenerative diseases and musculoskeletal conditions.
"I think the sky is the limit," said Dr. Srinivasan Mukundan Jr., PhD, medical director of MRI at BWH. "This additional field strength should really facilitate better imaging in many areas, but there will have to be considerable work done before we see any fruits of those efforts."
According to plan
BWH is a 793-bed nonprofit teaching affiliate of Harvard Medical School and a founding member of the Partners HealthCare system. It has more than 4.2 million annual patient visits and approximately 46,000 inpatient stays, and boasts more than 3,000 researchers, including physician-investigators, biomedical scientists, and faculty who are supported by approximately $666 million in funding.
The May 20 installation in BWH's Building for Transformative Medicine took most of the day to complete. It started by lowering the 25-ton investigational device (Siemens Healthineers) into a specially designed access hatch to a loading platform below. It eventually was pushed down a service corridor, which took several hours even with the hallway specifically designed in advance to accommodate the scanner's size and width. Finally, the staff connected the electronics.
 The scanner was detached from the crane, moved into the building, and slowly pushed down the service corridor into its specially designed room. The process took several hours. All images and video courtesy of BWH.
The scanner was detached from the crane, moved into the building, and slowly pushed down the service corridor into its specially designed room. The process took several hours. All images and video courtesy of BWH. Flooring was constructed to accommodate the installation of the new device and removal of old equipment.
Flooring was constructed to accommodate the installation of the new device and removal of old equipment."Having an MRI with a high-field magnet is something that the institution has thought about for a number of years," Mukundan said. "We like to say we built the building around the ultrahigh-field room. It is one of the cornerstone features of the building."
Only a handful of institutions have had a whole-body ultrahigh-field scanner for any reasonable length of time. Mukundan speculated that clinical clearance from the U.S. Food and Drug Administration (FDA) for 7-tesla MRI, if and when it does come, will be based in part on the modality's improved signal-to-noise ratio and advanced physics, which allow clinicians to view structures that are considerably smaller than what is seen with 1.5- and 3-tesla magnet strengths.
If the FDA clears 7-tesla MRI for clinical use, BWH then plans to apply to the state of Massachusetts to use the technology for clinical purposes.
"We are a determination of need (DON) state, so the [Massachusetts] Department of Public Health would grant us, if they saw fit, a DON," Mukundan said. "At that point, we could then use the device clinically. So there are two steps that need to be completed before we can offer this [scanner] clinically."
Time-lapse video shows the installation of the 7-tesla MRI system.
Clinical targets
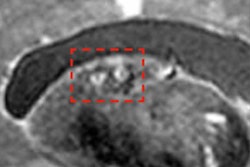
Neurodegenerative diseases such as multiple sclerosis and epilepsy and musculoskeletal conditions that involve the cartilage, muscle, and fascia of the knee joint are initial targets for 7-tesla MRI.
"In the study of multiple sclerosis, you can see a central vein in plaque, which pathologists see clinically on biopsy specimens. Those [central veins] are not clearly seen at 3 tesla," Mukundan said. "Another area is in epilepsy where some patients have refractory disease to medical therapy, and we cannot identify a lesion at 1.5 tesla or 3 tesla."
For epilepsy, the identification of a lesion in the brain -- previously not visible on standard MRI -- could allow for surgical treatment when drug therapy is ineffective, he said. The clinicians also expect 7-tesla MRI to offer new insights into traumatic brain injury and more detailed understanding of metabolic pathways in the brain.
"Another area that will clearly benefit is cartilage imaging, where we have ultrashort echo times that can be achieved with this device," Mukundan said. "That will allow us to better characterize lesions within the joint itself."
Patient tolerance
Of course, the choice to use 7-tesla MRI clinically would depend on risk-benefit deliberations and whether a patient would benefit from an ultrahigh-field scan. Early research has found that patients tolerate the 7-tesla magnet strength with minimal, if any, side effects, much the same as minor reactions during 1.5- and 3-tesla scans.
In fact, a 2016 study from researchers at University Hospital Essen in Germany found that a few patients experienced some balance issues after a 7-tesla MRI scan. The condition dissipated within minutes.
"There are some people who have had reported issues of dizziness or unusual sensitivities and such, as they are placed [in the device]," Mukundan said. "There is no clear data regarding anything that is lasting, but I'm speaking based on what is in the literature, rather than in terms of actual practice."
Radiologist training
Given the significantly greater detail of MR images provided by 7 tesla, will radiologists need to undergo more training to know what to look for, or will the ultrahigh-field magnet strength make interpretations easier for readers?
"I think the answer is 'yes' to every element," Mukundan said. "Yes, it will be easier; yes, it will be harder; and yes, we will have training. It is all of the above. We all need to learn to reset our expectations as technology improves, but it is clearly an iterative process with everybody."
One reason why Mukundan believes 7-tesla MRI is such an important technology is the ability to see abnormalities and internal processes more clearly, which will translate into better research and better care.
"What we want to do is apply [7-tesla MRI] technology where it may be beneficial and not apply it for technology sake alone," he added. "That is why it will take some time to mature and come to fruition."