Japan's Ministry of Health, Labor, and Welfare is following suit with regulatory agencies in the U.S. and Europe in asking four makers of gadolinium-based contrast agents (GBCAs) for MRI scans to revise warning text on their product labeling information.
The ministry's November 28 edict mandates that the wording on GBCA packaging be changed to advise users on how previous studies have found "high signal intensity in the cerebellar dentate nucleus and globus pallidus on unenhanced T1-weighted MR images" and that gadolinium was "detected in autopsied brain tissues in patients who received a GBCA several times." The agency is also requiring the insert to state that the "necessity of MRI scan using gadolinium-based contrast agents should be determined carefully."
The requirements target linear GBCAs gadodiamide (Omniscan, GE Healthcare), gadopentetate dimeglumine (Magnevist, Bayer HealthCare), and gadoxetate (Eovist, Bayer HealthCare), as well as macrocyclic GBCAs gadobutrol (Gadovist, Bayer), gadoteridol (ProHance, Bracco Imaging), and gadoterate meglumine (Dotarem, Guerbet).
In making its decision, the ministry followed Japan's Pharmaceuticals and Medical Devices Agency (PMDA), which issued a summary of its investigative results, citing 74 GBCA studies from around the world.
GBCA debate
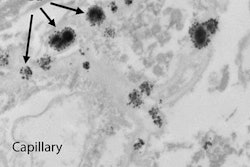
Japan can take credit for bringing the issue of gadolinium retention in brain tissue to the forefront. It was Dr. Tomonori Kanda, PhD, and colleagues from the Teikyo University School of Medicine in Tokyo who suggested in a December 2013 study the possibility that a toxic component of gadolinium MRI contrast may remain in the body long after it is injected, even in patients with normal renal function.
Their research ignited debate and a multitude of studies on the possible adverse effects of minute traces of gadolinium retained in the body and also whether gadolinium deposition can cause mild to severe health issues in patients, either shortly after an MRI scan takes place or years later.
Last month, the gadolinium controversy received widespread public attention when action film star Chuck Norris and his wife Gena filed a lawsuit against 11 companies involved in the manufacture and distribution of GBCAs. The couple alleges that the contrast agents are responsible for Gena's severe health problems and are seeking monetary damages of more than $10 million.
The changes in Japan follow similar concerns and recommended restrictions for GBCAs in the U.S. and Europe. In September, the U.S. Food and Drug Administration's (FDA) Medical Imaging Drugs Advisory Committee (MIDAC) voted overwhelmingly to recommend that the agency revise prescribing information for MRI GBCAs to include a warning about gadolinium retention in certain organs and tissue.
The MIDAC warning would note that minute traces of gadolinium may occur in slightly greater amounts with linear GBCAs, compared with macrocyclic agents, and recommend ways to minimize gadolinium retention for certain patient populations that may be at greater risk.
In March, the European Medicines Agency's Pharmacovigilance Risk Assessment Committee (PRAC) recommended that four GBCAs be pulled off the market due to concerns about gadolinium remaining in the body years after scans occur.
By July, however, the PRAC adjusted its earlier decision by allowing the use of gadobenate (MultiHance, Bracco) for liver imaging provided that it meets an important diagnostic need. The same guideline was set for gadoxetate disodium (Primovist in Europe, Eovist in the U.S., Bayer).
The PRAC maintained its position on the three other linear GBCAs, continuing to recommend the suspension of gadodiamide (Omniscan), gadopentetate dimeglumine (Magnevist), and gadoversetamide (Optimark, Guebert).
One company already is moving to adhere to the new Japanese requirement. Guerbet reported that it is immediately proceeding to make the requested revisions in its packaging for gadoterate meglumine, which is marketed in Japan as Magnescope. The company added that it will "continue to work with health authorities to further understand the mechanisms and consequences of gadolinium deposition in tissue."