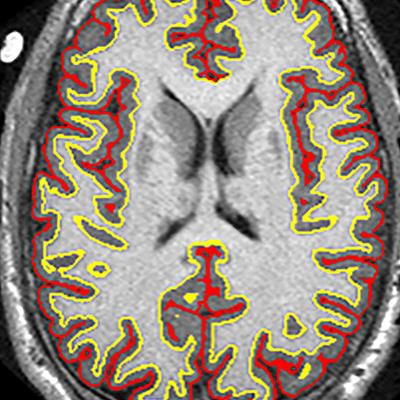
MR images from more than 200 patients with progressive multiple sclerosis (MS) show that a promising anti-inflammatory drug can decrease the progression of brain atrophy by 48% compared with a placebo, according to a study in the August 30 issue of the New England Journal of Medicine.
Over the course of the 96-week, phase II study, patients with progressive MS who took the anti-inflammatory drug ibudilast maintained approximately 2.5 mL more brain tissue than subjects taking a placebo. The findings, at the very least, offer some hope for these MS patients, who have few treatment options. The data could also provide insight for researchers into how to proceed with similar clinical trials.
"There are two big messages for the radiological community. One is that whole-brain atrophy does appear to be a useful metric for phase II trials with progressive MS," said principal investigator Dr. Robert Fox, vice chair for research at the Neurological Institute at the Cleveland Clinic. "And, with diffusion-tensor imaging, magnetization transfer ratio, and optical coherence tomography, cortical thickness or cortical atrophy appears to be a robust measure of tissue injury, progression of that injury, and therapeutic response with ibudilast."
MS in stages
Multiple sclerosis manifests as a gradual decline in neurological function. The early stages of MS present as a relapsing and remitting condition that attacks the protective myelin sheath of cells in the brain and spinal cord. Over the course of some 10 to 20 years, the disease gradually worsens and becomes progressive MS.
 Dr. Robert Fox from the Cleveland Clinic.
Dr. Robert Fox from the Cleveland Clinic.While previous research has linked the relapsing and remitting of MS to newly formed lesions that can be observed on MRI, there is little knowledge about the physiology of progressive MS.
"We do not know exactly what drives that, but we do know the therapies for relaxing or remitting MS tend not to work very well in progressive MS," Fox said.
One possible therapy is ibudilast, which is being developed as MN-166 by biopharmaceutical firm MediciNova in La Jolla, CA. In March 2016, the U.S. Food and Drug Administration (FDA) approved the company's request for "fast track" designation of the oral agent for progressive MS. MediciNova has several trials underway in which ibudilast is being investigated for MS, as well as for amyotrophic lateral sclerosis (ALS).
In one previous clinical trial, ibudilast's anti-inflammatory properties were assessed among patients with relapsing or remitting MS. While ibudilast did not stifle the formation of new lesions, it did slow the progression of brain atrophy in a dose-dependent fashion.
"Based upon that [finding], it got people to thinking," Fox told AuntMinnie.com. "If [ibudilast] does slow the progression of brain atrophy without decreasing the number of new lesions, maybe it could be useful in progressive MS where we do not have very good therapies, there is a gradual decline in neurological function, and where we see progressive brain atrophy as well."
Random doses
The current study enrolled 255 patients between 21 and 65 years of age from 28 U.S. sites. The subjects were diagnosed with either primary progressive or secondary progressive MS (NEJM, August 30, 2018, Vol. 379:9, pp. 846-855).
Of the total group, 129 subjects were randomly assigned to receive up to 100 mg of ibudilast orally (10 10-mg capsules) per day, while 126 patients received placebo pills in two or three divided doses per day. Eleven patients withdrew from the trial without sufficient imaging results for analysis. A total of 108 patients (84%) in the ibudilast group and 112 (89%) in the placebo group completed the trial.
The researchers assessed each patient's clinical disability based on the Expanded Disability Status Scale (EDSS) every 24 weeks. At the same time, subjects underwent an MRI scan on a 3-tesla scanner (Magnetom Trio/Prisma or Skyra, Siemens Healthineers; or Excite 12x, GE Healthcare), as well as optical coherence tomography (OCT). Two independent readers evaluated the thickness of the retinal nerve-fiber layer on OCT and measured the rate of brain atrophy based on parenchymal fraction.
Minimized loss
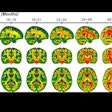
While all patients experienced atrophy, the brains of the patients with progressive MS in the placebo group shrank on average 2.5 mL more over two years than those in the ibudilast group, the researchers found. The rate of change in the brain parenchymal fraction was 0.0019 units of atrophy per year with the placebo, compared with 0.0010 units per year with ibudilast. That difference of 0.0009 was deemed statistically significant (p = 0.04).
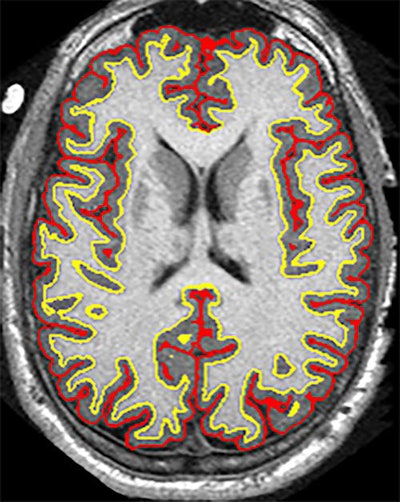 The trial results suggest that the anti-inflammatory drug ibudilast may slow brain atrophy caused by progressive MS. Image courtesy of Dr. Robert Fox and the Cleveland Clinic.
The trial results suggest that the anti-inflammatory drug ibudilast may slow brain atrophy caused by progressive MS. Image courtesy of Dr. Robert Fox and the Cleveland Clinic.There was a greater, but not statistically significant, overall rate of adverse events with ibudilast (119 events, 92%), compared with the placebo (111 events, 88%) (p = 0.26). Among that total were 20 serious adverse events (16%) with ibudilast and 24 serious events (19%) with the placebo (p = 0.46).
The most significant incidents and differences between the two regimens involved ibudilast's association with gastrointestinal issues, such as nausea, diarrhea, abdominal pain, and vomiting, as well as headache and depression.
While an adverse event percentage of 92% for ibudilast might appear high, Fox said the result should be viewed in context. When patients are followed over 96 weeks, the investigators are likely to record a variety of health issues.
"In truth, everyone has [health issues] over a two-year period, which could be recorded as an adverse event," he explained. "The fact that [the adverse event rate in this study] is not 100% is really surprising. The relative difference between the placebo and ibudilast is very, very small."
The researchers also found a "bit of an imbalance," with a higher rate of neck, arm, and leg pain with the placebo versus ibudilast, Fox said. That side note could suggest another use for the drug.
"This might be a slight suggestion in support of ibudilast having some activity in pain syndromes including pain seen in progressive MS," he speculated.
Data mining
As for future research with ibudilast, Fox suggested a couple of paths. For one, the current study "provides a compelling argument to take ibudilast into a phase III trial," he said. "It provides a compelling argument from efficacy, in terms of the activity on brain atrophy, and from a safety and tolerability [standpoint], where it was quite safe and reasonably tolerated."
Given the relatively small sample size of 244 patients used in the imaging analyses, he noted the need for more research before ibudilast could be considered for regulatory clearance.
"There is also a tremendous amount of data in this study that will be mined to understand progressive MS and understand how to conduct progressive MS trials in a more efficient and effective way," he said. "If we can speed up phase II trials, we can screen drugs more quickly, and we can identify effective therapies in a more efficient fashion."
The U.S. National Institute of Neurological Disorders and Stroke supported the study.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)