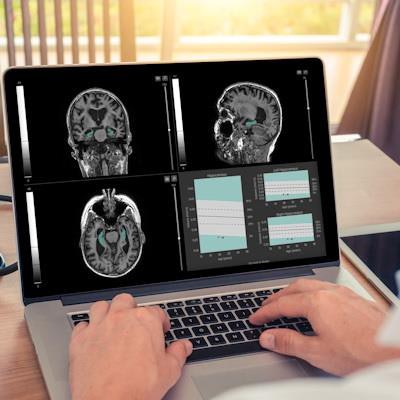
Medical technology company Qynapse said it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its QyScore brain MRI analysis software.
QyScore is designed to quantify markers of disease such as brain atrophy and white-matter lesions on MR images and then generate a report of the results within minutes. Part of the analysis includes quantifying longitudinal changes to regions of interest in the brain.
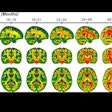
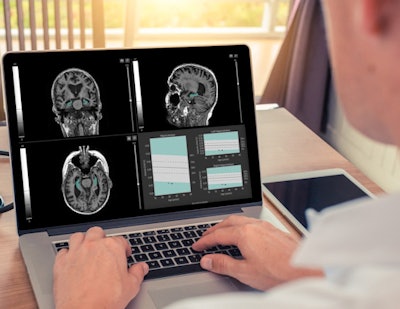 QyScore software. Image courtesy of Qynapse.
QyScore software. Image courtesy of Qynapse.The software is compatible with routine clinical workflow and can support physicians as they make their differential diagnosis for neurodegenerative diseases such as Alzheimer's, as well as monitor drug efficacy and safety, Qynapse said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)