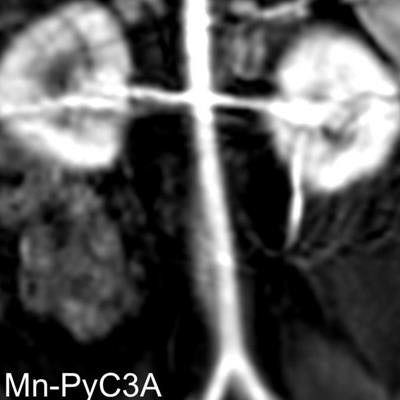
An MRI contrast agent based on manganese shows promise as an alternative to gadolinium-based contrast agents (GBCAs), according to research published in Investigative Radiology.
The findings are good news for patients with chronic kidney disease who need MRI, since gadolinium has been associated with causing nephrogenic systemic fibrosis (NSF) in renally impaired patients and remaining in the brain, bones, and skin.
Researchers from Massachusetts General Hospital (MGH) and Harvard Medical School, both in Boston, are developing the new agent, called Mn-PyC3A, which is based on manganese, an element found in nuts, legumes, seeds, and whole grains that can be easily processed and eliminated by the body. Mn-PyC3A has magnetic properties similar to gadolinium without the toxicity, principal investigator Eric Gale, PhD, said in a statement released by MGH.
Gale and colleagues used PET-MRI to compare Mn-PyC3A with an older manganese-based agent called Mn-DPDP, which is for liver imaging but is no longer available. The PET-MRI exams visualized how both agents moved through and were eliminated from the body. The team found that residual manganese from the Mn-DPDP injection remained in the bones, salivary glands, liver, and gastrointestinal tract, but manganese injected via Mn-PyC3A was quickly eliminated -- primarily through the kidneys -- and did not accumulate in any tissue.
The group also compared manganese and gadolinium retained in tissues seven days after equal doses were administered to renally-impaired rats. Elimination of manganese was more efficient than gadolinium at that seven-day checkpoint, according to the researchers.
"Clinical GBCAs are eliminated only through the kidney, and thus remain in renally impaired patients for longer periods resulting in increased gadolinium exposure," lead author Iris Yuwen Zhou, PhD, said in the MGH statement. "Our imaging data shows how for Mn-PyC3A, the liver compensates for diminished renal function and ensures rapid and complete elimination of Mn-PyC3A."




















