Portable MRI developer Hyperfine has secured U.S. Food and Drug Administration (FDA) clearance for the latest version of its Swoop system’s Optive AI software.
This is the tenth version of the software and features improvements in image quality for ultra-low-field MR imaging, the company said. The software enhances each stage of the imaging process, from noise cancellation and image acquisition to reconstruction and postprocessing, with algorithms applied across all sequences, Hyperfine said.
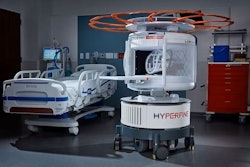
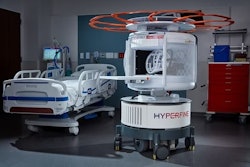
 Hyperfine's AI-powered Swoop portable MR brain imaging system.Hyperfine
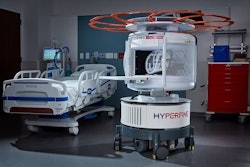
Hyperfine's AI-powered Swoop portable MR brain imaging system.Hyperfine
During product testing earlier this year, some clinical sites reported that the image quality of the Swoop system is approaching that of conventional 1.5 tesla MRI scanners, according to the company. Hyperfine plans to roll out Optive AI in the third quarter of 2025.