The U.S. Food and Drug Administration (FDA) has expanded use of Polarean's Xenoview to include children starting at age 6.
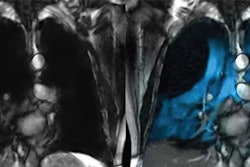
A hyperpolarized contrast agent for MRI, the xenon (Xe)-129 gas blend is an inhalant used in the evaluation of lung ventilation in adults and pediatric patients starting at age 12. Expanding the indication to include patients ages 6 and older will increase the number of those eligible by approximately one million, according to the firm.
The FDA supplement approval also includes the introduction of new Xenoview dose delivery bag sizes tailored for smaller lungs of younger patients and corresponding updates to the HPX Polarisation Measurement Station to measure various bag sizes.
In Polarean's announcement, CEO Christopher von Jako, PhD, said a controlled U.S. market release of the pediatric dose delivery bags is planned for later this year, beginning with Cincinnati Children’s Hospital. Pediatric pulmonologist Erik Hysinger, MD, emphasized the role of the product for children with chronic lung diseases, such as cystic fibrosis, asthma, bronchopulmonary dysplasia, and inflammation following bone-marrow transplant.