Boston Scientific announced that its TheraSphere radioembolization treatment is safe and effective for patients with hepatocellular carcinoma (HCC), according to the results of a trial presented March 25 at the annual meeting of the Society of Interventional Radiology.
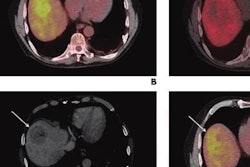
The study evaluated the safety and efficacy of therapy with TheraSphere yttrium-90 (Y-90) glass microspheres using a dosing method known as multicompartment dosimetry, which maximizes the dose of Y-90 reaching the tumor while minimizing the radiation dose that reaches normal liver tissue.
Imaging software was used retroactively to calculate the dose delivered within each patient's liver tissue. Data confirmed treatment was safe and well-tolerated, with only 4.8% of patients experiencing adverse events, defined in the primary endpoint as hyperbilirubinemia grade 3 or higher.
TheraSphere was approved by the U.S. Food and Drug Administration earlier this month for the treatment of HCC. The treatment is the only radioembolization technology in the U.S. indicated for the treatment of unresectable HCC, the company stated in a release.
Approximately 32,000 new cases of HCC will be diagnosed in the U.S. in 2021, according to American Cancer Society estimates.