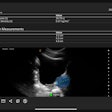
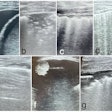
Computer-aided detection (CAD) software developer QView Medical said the U.S. Food and Drug Administration (FDA) has approved its premarket approval (PMA) application for QVCAD, a CAD software package for automated breast ultrasound (ABUS) studies.
The FDA has approved QVCAD for concurrent reading of ABUS studies. Based on deep-learning technology, QVCAD presents CAD results -- including the firm's C-thru Navigator image and CAD marks indicating the regions of interest -- simultaneously with the original ABUS image, according to the vendor.
QView said it will showcase QVCAD in its booth at next week's RSNA 2016 meeting in Chicago.