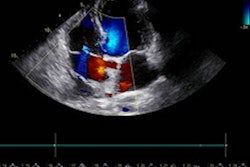
Dyad Medical's Echo:Prio echocardiography image analysis software has secured U.S. Food and Drug Administration (FDA) 510(k) clearance.
Available on the firm's Libby cloud- and artificial intelligence (AI)-based cardiac imaging analysis platform, Echo:Prio analyzes echocardiography images and serves as a decision-making support tool for index quantification of cardiac function, according to the vendor.
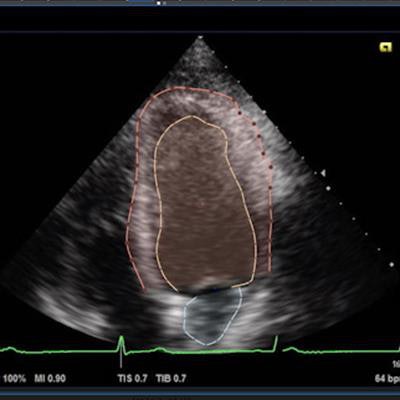
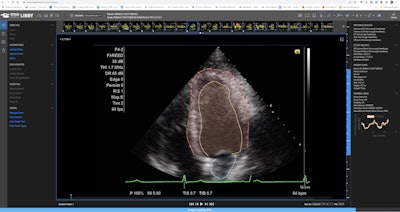 Dyad Medical's Echo:Prio AI-based echocardiography analysis software. Image courtesy of Dyad.
Dyad Medical's Echo:Prio AI-based echocardiography analysis software. Image courtesy of Dyad.It can be used from any location on any device and to provide an immediately available second opinion during interpretation, according to Dyad.