Pulmonary Embolism
Clinical Findings
No single, or combination of, clinical findings is either specific or sensitive enough to diagnose or exclude PE. Symptoms of pulmonary embolism include tachypnea (most common), tachycardia, hypoxia, pleuritic chest pain, hemoptysis, and atrial fibrillation. Massive PE may be associated with cor pulmonale and the ECG may show right axis deviation, P-pulmonale, RBBB, or other evidence of right heart strain. A normal arterial blood gas does not exclude the presence of a PE. Although PE occurs most commonly from deep venous thrombosis in the lower extremity, about 10% arise from clot in the upper extremity primarily associated with an indwelling catheter.
Risk Factors
In the PIOPED study, 92% of the patients with pulmonary embolism had at least one of
the following risk factors [R]: immobilization,
recent surgery, underlying malignancy, history of deep venous thrombosis or pulmonary
embolism, estrogen use, pre-existing cardiac disease. In patients without prior
cardiac or pulmonary disease only immobilization and surgery are significant
discriminating predisposing factors.
| Predisposing Factors: Patients with no previous cardiac or pulmonary disease [R] |
||
|
PE (n=117) |
No PE (n=248) |
|---|---|---|
| Immobilization | 66 (56%) | 81 (33%) * |
| Surgery | 63 (54%) | 78 (31%) * |
| Malignancy | 27 (23%) | 38 (15%) |
| Thrombophlebitis, Ever | 16 (14%) | 19 (8%) |
| Trauma - Lower Extremities | 12 (10%) | 25 (10%) |
| Estrogen Use | 10 (9%) | 26 (10%) |
| Stroke | 8 (7%) | 9 (4%) |
| Postpartum within past 3 mo. | 5 (4%) | 8 (3%) |
| * p < 0.001 | ||
Laboratory Findings:
None are suficiently diagnostic of PE for clinical use. However, D-dimer measurements of less than 500 micrograms/L could be used reliably to exclude PE in patients with an abnormal but not high-probability (inconclusive) lung scan [R]. On the other hand, normal oxigenation is not reliable to exclude PE [R].
Radiographic Findings:
CXR
The most common radiographic finding in patients with pulmonary embolism is atelectasis and/or parenchymal opacity. When looked for in the entire PIOPED population, it is found in at least 51% of patients with PE [R]. In a sub population of patients without prior cardiac or pulmonary disease, this finding occurred in 68% of those with PE and 48% of those without PE [R]. Pleural effusions within the affected hemithorax occurred in about 35% of patients with pulmonary embolism in the PIOPED study [R]. The majority of these pleural effusions were small, producing only costophrenic angle blunting. Occasionally, plain films may demonstrate findings such as Westermark's sign (local pulmonary oligemia, 11% of hemithoraces with PE), Fleischner's sign (regional oligemia in the presence of an ipsilateral enlarged pulmonary artery), and Hampton's Hump (a wedge-shaped pleural based density, 23% of hemithoraces with PE) [R].
CXR Findings in PIOPED Patients [R] |
|||
|
All pts |
Pts with PE |
Pts without PE |
|---|---|---|---|
Fleischner Sign |
216 (20.3%) |
77 (20.1%) |
139 (20.5%) |
Pulmonary Edema |
176 (16.6%) |
38 (10%) |
138 (20%) |
Normal |
166 (15.6%) |
45 (12%) |
121 (18%) |
Enlarged Hilum |
68 (6.4%) |
26 (7%) |
42 (6%) |
Enlarged Mediastinum |
50 (4.7%) |
18 (4.7%) |
32 (4.7%) |
COPD |
41 (3.9%) |
13 (3.4%) |
28 (4.1%) |
Helical CT (See discussion of PE in Chest Review Manual)
Angiography
Although considered the gold standard, angiography may not always detect the presence of emboli. Some indirect angiographic evidence for the presence of emboli is also non-specific. V/Q scans can provide an important "road map" to angiographers. Up to 95% of patients have been shown to have a PE ipsilateral to the abnormal side identified on V/Q scanning [1]. However, if the abnormally perfused segment on the V/Q scan appears normal at angiography, complete evaluation of the remainder of the lungs for the presence of pulmonary emboli is warranted as about 5% of patients can have isolated contralateral embolism [1].
Scintigraphic Examination
On V/Q scanning, a pulmonary embolism is characterized by a ventilation-perfusion mismatch: An area of normal ventilation corresponding to a segmental wedge-shaped area of decreased or absent perfusion which extends to the surface of the lung. Although PE typically has little or no effect on ventilation, if the scan is performed soon after a large (lobar) embolism has occurred (within 4 to 6 hours), rarely the vascular insult may induce reflex bronchoconstriction producing a matched ventilation-perfusion defect on both gas and aerosol ventilation studies [10].
In experimental animals, only 1.5% of emboli induced bronchoconstriction which lasted only a few hours. Bronchoconstriction is likely secondary to local hypocapnia (decreased carbon dioxide tension) and the release of serotonin, histamine, and other substances from platelets at the site of the embolus and it is a transient phenomena. Bronchoconstriction should be considered when multiple matched perfusion defects are seen in a patient without a history of airway disease and there has been only a short interval since the onset of symptoms. Follow-up ventilation study in these patients in 18 to 24 hours will demonstrate resolution of the ventilation abnormalities.
A NORMAL ventilation-perfusion scan essentially excludes the possibility of recent significant pulmonary embolus. The false-positive rate of high probability scans is only about 10%. In general, emboli are more frequent in the lower lobes due to the greater blood flow. Emboli are also frequently multiple (in 90%) and bilateral (in 85% of cases). Thus, unilateral decreased/absent perfusion to one lung is uncommonly the result of pulmonary embolism.
Injection of Tc-99m MAA into the veins of the feet may help to reveal a DVT. Venous obstruction or collateral flow can be seen. Images recorded several minutes after the injection may also show "hot spots" where the Tc-99m MAA is adherent to thrombus in the vein.
Interpretation is based on determining number of defects and descriptors of each one (see below).
Defect Descriptors, as Used in PIOPED:
V/Q ratio: Perfusion defect that has any ventilatory abnormality of comparable size is called a V/Q Match. If the perfusion defect has no corresponding ventilatory abnormality, or if it is either much more sever or larger than ventilatory abnormality, it is considered a V/Q Mismatch. NOTE: Any degree of perfusion decrement (NOT only the ones with completely absent perfusion) should be considered a perfusion defect. It is presumed that a partially occluding embolus could create a perfusion defect with perfusion that was diminished, but not absent. In fact, the pulmonary artery must be narrowed by at least 90% of its normal diameter before perfusion distal to the obstruction is reduced.
Size: Determining the size of a defect can be difficult. A knowledge of segmental anatomy is essential, and use of a schematic chart is encouraged to increase reading reproducibility and interobserver agreement (R). Note that, in general, the actual size of a perfusion defect is UNDER estimated by exam interpreters [13]- most likely due to shine through of radioactivity in contiguous segments [16]. In one study, only 44% of segmental defects were correctly identified as being greater than 75% of a segment [13]. Also- perfusion defects in the medial basal segment of the right lower lobe are often not detectable in any view [13]. Interobserver agreement regarding defect size is poor (about 40%) and even intraobserver agreement is only moderate (about 56%) [15].
* Small defect (small subsegmental): Less than 25% of a segment.
* Moderate defect (moderate subsegmental): > 25%, but < 75% of a segment.
* Large defect (segmental): Greater than 75% of a segment.
Vascular segmental distribution: A segmental defect (independent of size) is a perfusion abnormality which may be triangular or rectangular shaped, periphery based, and specially located within one or several vascular segments. Nonsegmental defects do not conform to segmental vascular anatomy and unlikely to represent PE. Because the term "segmental" is also used in sizing of defects, we prefer to call defect vascular or nonvascular in appearance and distribution.
Defect Descriptors, Identified in Studies Other Than PIOPED:
"Segmental Equivalent" Sizing: The moderate segmental defect is counted as 0.5 of a large segmental defect (two moderate sized segemental defects are therefore equivalent to one large segmental defect). All moderate and large defects can be than added up to result in total segmental equivalent units. (Example: 3 moderate segmental defects and 1 large = 2.5 segmental equivalents)
"Stripe Sign": A thin line (stripe) of activity (perfusion) at the pleural surface of a perfusion defect. The finding is associated with underlying emphysema and is likely related to spared perfusion in the cortex of the lung [6]. Iin the PIOPED study only 7% with a stripe sign actually had a pulmonary embolism corresponding to that segment [6]. Hence, in the absence of other perfusion abnormalities, the finding is considered low probability for PE. In cases in which there is a high clinical concern for pulmonary embolism, SPECT images may help to better define the defect [R].
"Triple Match": A matching perfusion, ventilation, and CXR abnormality is referred to as a "triple match". [8]. The overall prevalence of PE in all lung zones with triple matches was 26% in the PIOPED population [8]. There were no significant differences between the size of the matching V/Q defects and chest radiographic opacities and the prevalence of PE [8]. The prevalence of PE in small regions (less than 25% of a zone) with triple matches was 27%, compared with 21% in large regions (over 75% of a zone) with triple matches [8]. However, there was a difference in prevalence of PE between lung zones- pulmonary embolism was significantly more common in lower lung zone triple matches (upper - 11%; middle - 12%; lower - 33%) [3,8]. Pulmonary embolism was significantly more common in lower lung zone triple matches [8]. Therefore, a triple-match in the upper or middle lung zones is considered low probability for PE, but a triple-match in the lower lung zone should be interpreted as intermediate probability [8].
Interpretation Criteria
Interpretation is based on fulfillment of specified criteria. Several criteria sets are
available, but the most commonly used ones are Biello's and PIOPED. The original PIOPED
criteria is the only set that was tested prospectively. Modifications to PIOPED criteria
have been suggested on the bases of retrospective PIOPED data review.
Interobserver variability in scan interpretations is good when using the PIOPED
criteria [18]. The most recent
publication of Modified PIOPED Criteria appeared in the form of Society of Nuclear
Medicine procedure guideline [R]:
High Probability (80-100% likelihood for PE [12]):
1. Greater than or equal to 2 large mismatched segmental perfusion defects or the arithmetic equivalent in moderate or large and moderate defects. A high probability lung scan confirms a very high likelihood for pulmonary embolism and justifies treatment with anticoagulation (unless contraindicated) [18].
Caveat - It has been suggested that 2.5 mismatched large segmental defects (or the arithmetic equivalent) is a better threshold for calling a scan high probability, as it associated with a 100% probability of PE in the PIOPED population [12].
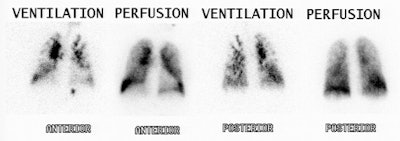
|
High probability scan for pulmonary embolism: The patient shown in the case below had undergone right knee prosthesis surgery and had a known lower extremity deep venous thrombosis. The patient had become acutely short of breath and a V/Q scan was ordered to evaluate for the presence of pulmonary embolism. The perfusion scan demonstrated multiple segmental perfusion defects of varying sizes within both lungs- consistent with "showering" emboli. |
|
|
Intermediate Probability (20-80% likelihood for PE [12]):
1. One moderate to 2 large mismatched perfusion defects or the arithmetic equivalent in moderate or large and moderate defects.
2. Single matched ventilation-perfusion defect with a clear chest radiograph [12].
Caveat - Single ventilation-perfusion matches are borderline for "low probability" and thus should be categorized as "intermediate" in most circumstances by most readers, although individual readers may correctly interpret individual scintigrams with this pattern as "low probability".
3. Difficult to categorize as low or high, or not described as low or high.
|
Intermediate probability scan for pulmonary embolism: The patient shown below had a single segmental perfusion defect in the superior ligular segment of the left upper lobe. Further evaluation was recommended to exclude a pulmonary embolism. A CT PE study confirmed the presence of a PE in this segment. No other emboli were identified. |
|
|
Low Probability (0-19% likelihood for PE [12]):
1. Perfusion defects matched by ventilation abnormality provided that there are: (a) clear chest radiograph and (b) some areas of normal perfusion in the lungs. Extensive matched V/Q abnormalities are appropriate for low probability, provided that the CXR is clear.
2. Any perfusion defect with a substantially larger chest radiographic abnormality.
3. Any number of small perfusion defects with a normal chest radiograph.
4. Nonsegmental perfusion defects (e.g., cardiomegaly, enlarged aorta, enlarged hila, elevated diaphragm).
5. Multiple matched V/Q abnormalities, even when relatively extensive, are low probability for PE [11]. The prevalence of PE in patients with extensive matched V/Q defects and no CXR abnormality was 14% (low probability).
|
Low probability scan for pulmonary embolism: The patient had a clear CXR and complained of SOB. The ventilation portion of the exam demonstrates markedly heterogeneous ventilation with central deposition of aerosolized Tc-DTPA consistent with COPD. There is a matching perfusion pattern. Extensive matched V/Q abnormalities are appropriate for low probability, provided that the CXR is clear. |
|
|
Normal:
1. No perfusion defects or perfusion exactly outlines the shape of the lungs seen on the chest radiograph (note that hila and aortic impressions may be seen and the chest radiograph and/or ventilation study may be abnormal).
V/Q Scan Interpretation Nuances:
"Gestalt Interpretation" is formed by an experienced reader based on detailed knowledge of the various published lung image interpretation algorithms, as well as clinical data, ancillary findings, and pathophysiological features of PE, which are integrated with the individual case presentation [17]. A gestalt interpretation can be more accurate than any individual criteria set [R]. The Gestalt interpretation takes into consideration the fact that among patients with no underlying cardiopulmonary disease, fewer mismatched segmental defects are required to give a particular specificity and positive predictive value [15]. For instance, a previously healthy 25 year old female on birth control pills with a normal CXR and a single large segmental ventilation-perfusion mismatch in the lower lung zones on V/Q scanning may be interpreted to have a high probability study by an experienced reader. The Gestalt interpretation has been shown to have good-to-excellent intra- and interobserver variability [17,18].
A single moderate sized V/Q mismatch would qualify for low probability in the original PIOPED schema. However, such a finding was found to harbor PE in 36% of cases in the PIOPED cases [12]. Hence, it has since been considered to indicate intermediate probability.
"Triple Match" in the upper and middle zones is suggested for low probability. If seen in the lower zone, it qualifies for intermediate probability.
"Stripe Sign" is suggested to indicate low probability- 93% of lung zones with the stripe sign had no PE present on angiography in the PIOPED study [11].
A single matched (any size) V/Q defect has a 26% incidence of PE in the PIOPED population, hence, it is suggested to be classified as intermediate probability [12].
Defects associated with pleural effusion: Ipsilateral pleural effusions can be found in up to 35% of patients with acute pulmonary embolism. In the PIOPED study, the majority of patients with PE and pleural effusions had small effusions which caused blunting of the costophrenic angle. When the pleural effusion caused an isolated perfusion defect congruent with the size of the pleural effusion, and when the pleural effusion was limited to the costophrenic angle, the incidence of PE was 25% [12]. Therefore, matching V/Q defects with a small effusion represent intermediate probability for PE. In patients with no prior cardiopulmonary disease and an effusion that was greater or equal to one-third of the hemithorax, no PE was found, (i.e., low probability) [3,R]. However, some authors outside the PIOPED group argue that any size perfusion defect should be considered intermediate probability in association with any size effusion [R].
Generalized CXR abnormality: A generalized abnormality on CXR (such as diffuse pulmonary edema or reticulonodular disease) may not cause the perfusion lung scan to be abnormal- in fact, up to 73% of patients with such findings can have normal or near normal perfusion images [15].
Clinical Probability of PE:
The actual incidence of PE is highly dependent upon the pretest (clinical) likelihood for the presence of pulmonary embolism. The utility of the V/Q is optimized when it is interpreted in conjunction with the clinical likelihood for PE. This was well demonstrated in the PIOPED [R]:
Clinical Science Probability |
||||
| Scan Probability | 80 - 100% | 20 - 79% | 0 - 19% | All Probabilities |
| High | 96% | 88% | 56% | 87% |
| Intermediate | 66% | 28% | 16% | 30% |
| Low | 40% | 16% | 4% | 14% |
| Near normal/ normal | 0% | 6% | 2% | 4% |
| Total | 68% | 30% | 9% | 28% |
PIOPED Summary:
The PIOPED study was a Prospective Investigation of Pulmonary Embolism Diagnosis that tested utility of V/Q scanning. All patients enrolled in the study were to have a V/Q scan and a pulmonary arteriogram. Unfortunately, a large number of patients with normal or low probability scans who were enrolled in the study did not undergo arteriograms as many physicians did not want to have their patients exposed to the risks of the procedure in light of the V/Q scan findings. Because of this, there was a selection bias towards patients with intermediate or high probability scans (prevalence of PE based upon arteriogram for patients enrolled in the study was 33%). Such a bias would tend to overestimate the sensitivity, but underestimate specificity of V/Q scanning. Agreement among the readers was excellent for high, very low, and normal scans (92-95%), but agreement for intermediate and low probability scans was not as good [15]. In patients with chronic obstructive pulmonary disease, the sensitivity of a high probability V/Q scan is significantly lower compared to patients with no pre-existing cardiopulmonary disease. In fact, as the complexity of the patients underlying cardiopulmonary disease increases, so does the likelihood that the scan will not be informative (intermediate probability) [15]. Using PIOPED criteria, intermediate probability V/Q scans occurred in 60% of patients with COPD, but in only 13% of patients with normal CXR's [15].
An analysis of the regional distribution of PE on angiography demonstrated that PE occurred more frequently in the right lung, compared with the left, and more frequently in the lower lung zones compared with the middle or upper zones.
False positives
Patients with prior pulmonary embolism can have persistent ventilation-perfusion abnormalities [15]. Pulmonary emboli resolve because of natural thrombolytic processes [15]. A residual perfusion defect can be found in 9% to 30% of patients and incomplete resolution may be more common in patients with underlying cardiopulmonary disease [15].
Among young non-smokers approximately 7% may demonstrate subsegmental perfusion defects and 3-4% lobular or segmental defects. As many as 10% of smokers may exhibit some type of perfusion defect. Xenon washout studies have also demonstrated mild delay in regional clearance and heterogeneity of clearance time in young smokers. These changes become progressively more severe and irreversible in long-term smokers.
Multiple nonsegmental, matched ventilation-perfusion defects can be seen following smoking of free based cocaine and snorting heroin. This may be related to the local vasoconstrictive and bronchoconstrictive actions of cocaine [9].
Bronchoalveolar lavage can also produce perfusion defects and lung scanning should be delayed at least 24 hours following the procedure to avoid mis-interpretations [7].
Post-scan clinical decisions
Recommendations regarding the further evaluation of patients, based upon the V/Q scan findings and clinical likelihood of embolism: [Radiologic Clinics of North America, July 1993, p. 856]
* Patients with normal studies require no further evaluation or treatment.
* Patients with a low probability scan, a low clinical likelihood, and no clinical evidence of DVT do not require anticoagulation or further workup.
* Patients with a low probability scan, and an intermediate or high clinical suspicion of embolism, should have serial ultrasound of the lower extremities to exclude DVT. If these studies are negative, then anticoagulation is not necessary, however, careful follow-up is recommended. In general, complications from PE are identified infrequently in patients with low probability V/Q scans [4]. Nonetheless, this same article recommended that these patients should subsequently undergo pulmonary angiography if the duplex exam was normal.
* Patients with an intermediate probability scan require ultrasound of the lower extremities. If these studies are negative, further evaluation with angiography may be warranted. The low morbidity (6%) and mortality (0.5%) associated with pulmonary angiography justifies its use in selected patients with suspected pulmonary embolism [3].
Duplex US has a sensitivity of 95% and a specificity of 99% for the detection of proximal vein DVT. In one study, DVT was detected in 21% of patients with normal lung scans, 15% with low probability scans, 30% with intermediate prob. scans, and 50% of patients with high prob. scans. The presence of a DVT in patients with an intermediate probability lung scan raises the likelihood of PE to 93% [4].
On follow-up, a change in the perfusion pattern can be seen as early as a few days following the event. Defects may become smaller and disappear, or large clots may fragment creating new peripheral defects. A scan performed as early as 5 to 10 days post-event frequently shows marked resolution of perfusion defects (except in the elderly, about 50% of defects will have resolved by 2 weeks in patients treated with heparin). Approximately 15% of patients will have a persistent perfusion defect at one year post event, although the abnormality is typically less well defined. Resolution of a scan defect is generally age dependent, with about 50% of patients less than 40 years old having complete resolution of defects, while few patients over the age of 60 years will have their scan return to normal. Emboli which lead to pulmonary infarction are also unlikely to have resolution of the perfusion defect. A 4 week follow-up exam is beneficial to serve as a baseline study should these patients become symptomatic in the future.
Treatment for pulmonary embolism
Treatment for PE most commonly consists of anticoagulation with heparin or coumadin. When properly diagnosed and treated, death attributed to pulmonary embolism is relatively uncommon (occurring in about 2.5% of cases) [2]. Thrombolytic agents (such as urokinase or tPA) are not routinely used in the treatment of pulmonary embolism. Such treatment is generally reserved for patients with large clot burdens (i.e., occlusion of greater than 50% of the pulmonary bed, or 25-30% in patients with pre-existing cardiac or pulmonary disease) or for patients who are hemodynamically unstable.
REFERENCES:
(1) Radiology 1999; Davey NC, et al. Ventilation-perfusion lung scintigraphy as a guide
for pulmonary angiography in the localization of pulmonary embolism. 213: 51-57
(2) N Engl J Med 1992; Carson JL, et al. The clinical course of pulmonary embolism. 326:1240-1245
(3) J Nucl Med 1995; Worsley DF, Alavi A. Comprehensive analysis of the results of the PIOPED Study. Prospective Investigation of Pulmonary Embolism Diagnosis Study. 36: 2380-2387
(4) Radiology 1994; Smith LL, et al. Pulmonary embolism: Confirmation with venous duplex US as adjunct to lung scanning. 191: 143-147
(5) Radiology 1994; Sostman HD, et al. Evaluation of revised criteria for ventilation-perfusion scintigraphy in patients with suspected pulmonary embolism. 193: 103-107
(6) Radiology 1992; Sostman HD, Gottschalk A. Prospective validation of the stripe sign in ventilation-perfusion scintigraphy. 184: 455-459
(7) J Nucl Med 1993; Chen CC, et al. Abnormalities on ventilation/perfusion lung scans induced by bronchoalveolar lavage. 34: 1854-1858
(8) J Nucl Med 1993; Worsley DF, et al. Detailed analysis of patients with matched ventilation-perfusion defects and chest radiographic opacities. 34: 1851-1853
(9) Semin Nucl Med 1993; Benson ML, Balseiro J. Multiple matched ventilation-perfusion defects in illicit drug use. 23: 180-183
(10) J Nucl Med 1994; Aug: 1351-57
(11) J Nucl Med 1993; Gottschalk A, et al. Ventilation-perfusion scintigraphy in the PIOPED study. Part I. Data collection and tabulation. 34: 1109-1118
(12) J Nucl Med 1993; Gottschalk A, et al. Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. 34: 1119-1126
(13) J Nucl Med 1992; Morrell NW, et al. The underestimation of segmental defect size in radionuclide lung scanning. 34: 370-374
(14) Semin Nucl Med 1991; Juni JE, Alavi A. Lung scanning in the diagnosis of pulmonary embolism: the emperor redressed. 21: 281-296
(15) Prog Cardiovasc Dis. 1994; Stein PD, Gottschalk A. Critical review of ventilation/perfusion lung scans in acute pulmonary embolism. 37: 13-24
(16) J Nucl Med 2002; Meignan MA. Lung ventilation/perfusion SPECT: the right technique for hard times. 43: 648-651
(17) J Nucl Med 2002; Hagen PJ, et al. How to use a Gestalt interpretation for ventilation-perfusion lung scintigraphy. 43: 1317-1323
(18) J Nucl Med 2003; Hagen PJ, et al. Comparison of observer variability and accuracy of different criteria for lung scan interpretation. 44: 739-744