PET Imaging General Topics
Physical Principles of PET imaging
Agents used in PET imaging are typically
produced in a cyclotron by bombarding a stable element with protons, deuterons, or helium
nuclei. The resulting isotope will contain excess protons and will decay by positron
emission. PET imaging then utilizes physiologic substrates labeled with these positron
emitting isotopes. The emitted positron travels only a minimal distance (about 2 mm
maximum distance for 18F) before it undergoes an annihilation reaction with the
production of two 511 keV photons which travel in a 180 degree direction of one another.
PET resolution is inherently limited by
several factors [40]. The positron emitted by the radioactive isotope has a
certain amount of energy and will travel a short distance within tissue prior to annihilation
[40]. This change in position between the origin of the positron and its site of
annihilation results in positron range blurring, limiting the spatial resolution
of PET imaging [31]. The maximal range a positron can travel in soft tissue is between 2 to 20
mm, however, only a small fraction have high enough energy to travel the maximal
distance [40]. For 18F (maximum
positron energy 0.64 MeV) the positron range causes a resolution limit of about
0.22 creating an imaging blurring of less than 1 mm [40]. For
the worst case scenario of 82Rb (maximum positron energy of 3.35 mEV)
the positron range leads to a resolution limit of 2.6 mm [40]. Resolution is
also limited by the fact that the angular
range of the two annihilation photons is not quite 180 degrees [31]. The spread is actually a Gaussian distribution about 180 degrees with a Full-Width Half-Maximum (FWHM) of
0.3 to 0.5
degrees (which translates to a resolution effect of approximately 3-5mm for the
100 cm detector separation of a typical whole-body scanner) [31,40]. Resolution and quantitative performance are also affected by the distance between
detectors (detector ring diameter). The closer the detectors are to each other and to the
source, the greater the resolution.
Unfortunately, the smaller the diameter of the scanner ring, the more likely scattered and random coincidences will be recorded. The primary limiting factor that determines the spatial resolution of a PET scanner is the size of the scintillation crystal (ie: the number of crystals used in the scanner) -- the smaller the crystal, the greater the number of detectors, and the better the spatial resolution [18].
The detectors themselves have an intrinsic spatial resolution which is quantified by the apparent width obtained for a collimated point source- the shape of the apparent width is called the point-spread function and it is often characterized by it's full width at half maximum (FWHM) [40].
PET cameras- Detectors/Crystals
All PET scanners use scintillation
technology in which an incident photon interacts with the detector producing a flash of
light that is detected by a position sensitive photomultiplier tube behind the crystal.
Dedicated full ring PET scanners contain thousands of detectors which permit very good
spatial localization- the systems resolution is limited by the size of the detector
crystal. The ideal PET detector would have a very high sensitivity for stopping the
incident photon. It would produce a short and very intense light flash (photofluoresence)
that would permit accurate energy measurement and limit detector dead time. The types of
crystals most commonly used in PET cameras include bismuth germinate oxide (BGO), lutetium
oxyorthosilicate (LSO), gadolinium orthosilicate (GSO), and thallium-doped sodium iodide - NaI (Tl).
The physical properties of the crystal will ultimately affect the performance of the PET
camera.
Bismuth germinate oxide (BGO) crystals are
generally used in conventional PET imaging systems. BGO crystals have a high stopping
power (high efficiency), high spatial resolution, and are 50% more efficient than
thallium-doped sodium iodide -- NaI (Tl) -- crystals. Most crystals are 3-6 mm thick and
they are not hydrophilic. The detection efficiency for 25 mm BGO crystal is
approximately 80% [31]. The spatial resolution approaches 5 mm, which nears the
theoretical limit of resolution. The disadvantages of BGO crystals are that they have a
much lower light output (15% of NaI (Tl) crystals), long photofluorescent decay times
(decay constant of 300 ns which limits count rates/coincidence timing resolution
[31]), and
poorer energy resolution than sodium-iodide crystals. Energy resolution of BGO is normally
worse than 20-25% in FWHM at 511 keV. A typical energy window for a BGO scanner
is 300-350 keV to 650 keV [18]. This poor energy resolution makes it difficult to
remove scattered events by energy discrimination. Therefore, lead-tungsten septa
are interposed between detector rings to reduce interplane scatter [18]. The coincidence time window is normally
set for 10-20 ns. The inferior time resolution causes larger accidental detections and
greater dead times. BGO detectors are best suited for imaging isotopes with long
half-lives such as F-18 and C-11.
Lutetium Oxyorthosilicate (LSO) crystals offer the best combination
of properties for PET imaging [2]. LSO has a higher effective Z
(number of protons per atom) and density compared to BGO which results in an
equal or higher detection
efficiency [3,31]. It has very good energy resolution (about 12% [21]- with a
typical energy window set to 425-650 keV), a short decay constant for good
coincidence timing (a decay constant of 40 ns and coincidence time window of 4.5
ns), and higher light output (five
fold more light compared to BGO crystals [31])
[2]. The coincidence time window is set to 4.5 ns. These characteristics enable image formation is less time when LSO crystals
are used [31]. The crystal is rugged and nonhygroscopic.
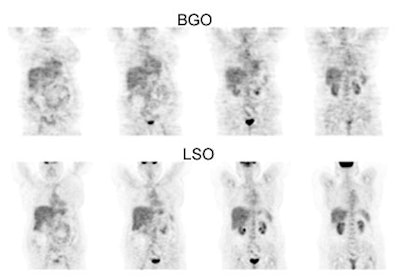
BGO versus LSO detector: The images below were acquired in 2D mode with a scan time of 5 min/bed emission and 3 min/bed transmission. The upper row of images were acquired on a BGO camera system and the lower row of images were acquired on a ECAT Accel LSO PET camera system. The data was reconstructed using normalized attenuation weighted OS-EM reconstruction. Image courtesy of Northern California PET Imaging Center, Sactamento, CA and CTI, The Power Behind PET. |
|
Gadolinium oxyorthosilicate (GSO) systems have a lower effective atomic number compared to BGO and LSO crystals- this permits gamma ray detection across a wide energy spectrum (from 28-35keV up to 511 keV) [31]. GSO also has improved energy resolution (10-15%) compared to BGO cameras which leads to a more narrow energy window and better scatter rejection [18,21]. They also operate with a narrow coincidence window of about 8 ns [18]. The decay constant is about 50-60 ns which compares favorably to LSO [21,31]. The light output from GSO is relatively low compared to LSO crystals [21], but is slightly more than BGO [31].
Lutetium yttrium orthosilicate (LYSO) system [63]: LYSO has an effective atomic number of 65; the density is 7.1 g/cm2, and the attenuation coefficient is 0.83 cm-1 at 511 keV [63]. The use of a fast, dense, and high light output scintillator such as LYSO leads to high sensitivity, reduced scanner dead time, and good spatial resolution [69]. The scintillation decay time is 42 ns and it has a light yield that is higher than BGO and similar to that of LSO [63]. The fast decay time leads to improved timing resolution and a narrower coincidence timing window- this in turn reduces the number of random events [63]. The higher light yield permits the use of smaller crystals within a block detector which results in improved spatial resolution (about 4.8 mm near the scanner center [69]) and good energy discrimination (allowing the energy discrimination window to be narrowed by raising the lower discriminator- typical energy range 440-665keV [69])- this has the effect of reducing the scatter fraction [63,69]. Small and average sized patients can be imaged using 1-2 minutes per bed position and 3 minutes for heavier patients with increased attenuation [69].
NaI (Tl) PET scanners (such as the CPET)
utilize curved detectors that improve spatial resolution. Although NaI (Tl) detectors have
a relatively low stopping power compared to other PET detectors, they demonstrate very
good energy resolution (11%) and have an excellent light yield [4]. This permits raising
the lower energy threshold to 435 keV (rather than 350 keV which is used in most
conventional BGO PET scanners) to limit scattered events without reducing true events [4].
The upper energy window is typically set to 590-665 keV [18]. The
coincidence time window is generally 8 ns which is also shorter than that for BGO systems
[4]. A shorter coincidence time window should improve counting characteristics [4]. The
system has a long crystal decay time (230 ns) compared to LSO systems (40 ns), but this is
shorter than the decay time for BGO (300 ns) [4]. NaI (Tl) systems rely on a 3-D
acquisition mode in order to achieve a high count density.
LaBr3 is a detector material that is being developed [40]. The material has high light output, a short decay time, and excellent energy resolution that permits effective scatter rejection (energy gate as high as 470 keV) [40]. The timing resolution is between 250-275 ps [40]. The stopping power is somewhat lower than LSO.
Time of Flight (TOF) PET imaging systems utilize cerium fluoride (CeF3) or barium fluoride (BaF2) crystals. These crystals have very short resolving times and coincidence localization can be obtained to within a fraction of a nanosecond, permitting improved sensitivity and signal to noise ratio. The coincidence time window is normally set for 2 ns or less. Unfortunately, these crystals typically have poor energy resolution and less light output than BGO crystals. The use of deeper detectors also degrades image resolution. The main advantage of TOF-PET is to improve statistical data, not spatial resolution. TOF-PET is best for imaging large objects with low contrast (due to the presence of less background noise) and for studying dynamic processes.
System dead time:
When a photon interacts with a
detector the crystal produces a light flash which is collected by several
photomultiplier tubes (PMT's). The photomultipliers tubes determine the enrgy
and spatial position of the event and this is followed by coincidence processing
[18]. The total time required to complete these tasks is the system dead time.
During this time, the system is unable to collect new incoming events which will
be lost [18]. System dead time can be reduced when using scanners with shorter
crystal scintillation times (such as LSO cameras) or by using a greater number
of PMT's [18].
Image Acquistion: 2D versus 3D
Compton scatter occurs when a photon undergoes and interaction which results in a lower energy and new trajectory [18]. Scatter removal is important because it will create image blurring [18]. The most ideal way to remove scatter would be to detect only 511 keV events- however, this requires systems with very good energy resolution and a narrow photo-peak window [18]. Unfortunately, this is not practical as most BGO PET systems have limited energy resolution and require wide energy windows (i.e.: scatter cannot be completely rejected by energy discrimination alone [55]) [18].
PET images can be acquired in either a 2-D or 3-D mode. To reduce the number of scattered photons in 2-D acquisitions, lead or tungsten septa are placed between the detectors to absorb scattered radiation (out of slice activity) [55]. The septa reduce the amount of scatter to 10-15% of the total counts acquired [3] and improve image contrast. However, the septa also reduce the sensitivity for unscattered photons [55] and random coincidence events (and some scattered events) will still be recorded and provide incorrect localization information which degrades the image. The number of random events generally increases with the dose of administered activity. A general rule of thumb for PET imaging using a BGO camera is that if there are sufficient counts to perform a study in 2-D mode, then that is the preferred scanning method. For BGO scanners, 2D acquisitions using the maximum allowable injected dose produce images with superior lesion detectability [34].
Random events: A random coincidence event occurs when photons from two separate annihilation reactions are detected within the timing window. The event will be localized along a path that does not correspond to the origin of the photons. |
|
When the septa are removed this is referred to as a 3-D acquisition and there is a large increase in the sensitivity of the detector [18]. In this type of acquisition, each individual detector is sensitive to radiation from a much larger area and the count-rate increases 5-6 fold compared to a 2D acquisition [3,18]. 3-D imaging can significantly reduce the amount of tracer activity needed for the exam or shorten the acquisition time [5]. 3-D scanning requires faster coincidence detection, more computer memory, and more time for image reconstruction [5,18]. Lutetium oxyorthosilicate (LSO), gadolinium oxyorthosiliacate (GSO), and NaI (Tl) PET scanners have physical properties which permit them to perform better than BGO cameras for 3-D image acquisitions [40,55]. In fact, when using an LSO camera, two-minute/bed 3-D position emission scans have been shown to detect all lesions seen on 4-min/bed position images, although exam quality will improve with longer acquisition times [35]. Because photon attenuation and scatter is higher in obese patients there is loss of true coincidences which degrades the signal-to-noise ratio [43,53]. This can particularly affect 3D acquisitions- a five minute per bed position scan appears to be adequate to detect lesions when using LSO imaging in obese patients (and a dose of 0.21 mCi 18F-FDG/kg) [43]. [55]
3-D scanning is often used for CNS imaging (even with a BGO scanner) because it is a small object containing a large amount of activity. Pediatric patients are also sometimes imaged with a 3D acquisition.
Disadvantages of 3-D scanning are:
1- The count rate limit of the scanner can be overwhelmed if too much activity is administered. With high count rates pulses can "pile-up" and the detector may become paralyzed due to dead time [40].
2- Increased random events: A random event occurs when photons from two separate annihilation events are detected within the preset coincidence time window and are recorded as a coincidence event- this produces low-level noise in the PET image [18]. The random coincidence rate increases with the amount of activity in the patient [40]. In 3-D mode, an increased number of random events will be detected and degrade the image. For a given total counting rate, the fraction of random events recorded will be greater when scanning in 3-D mode [1]. When scanning in a high counting rate environment, the random counting rate increases much more rapidly than does the true counting rate as a function of radioactivity in and near the field of view [1]. The true counting rate scales linearly with radioactivity in the field of view, while the random rate scales as the square of the radioactivity in and near the field of view [1]. The most common method of correcting for random coincidences is a real-time subtraction of a delayed coincidence channel, where one of the single-photon events has an arbitrary large time delay [45]. The rationale for this method is that a delayed coincident event cannot arise from a true or scattered coincidence event [45]. However, other methods of correction such as a reduced variance random estimation may result in an improved SNR [45].
3- Increased scatter: Scatter occurs when one (or both) annihilation photons are deflected (via a Compton interaction) within the body prior to detection- this results in an incorrect localization of the event and degrades the image [1]. In 3-D mode, the number of scattered events approaches 30% to 50% of all recorded events [51]. Because of this, for proper quantification, a scatter correction must be applied to 3-D data.
4- Increased number of bed positions: In 3D mode, the increase in system sensitivity that results from all events in the field of view (FOV) being seen by the scanner produces a sensitivity profile with a maximum in the center of the FOV. There is a rapid decrease in the profile on moving away from the peak [18]. For this reason, whole-body exams need to reduce the axial FOV to maintain a uniform count profile. This results in an increase in the number of bed positions to cover the same patient length [18].
Attenuation Correction
PET technology allows for attenuation correction of the images. This can produce a more accurate final image that may detect smaller lesions, especially when they are deep within the body [11]. The disadvantages of attenuation correction is that it requires more time for image acquisition and there is the potential to add noise to the image if the attenuation measurements become misaligned by patient motion [11].
Attenuation correction is performed to account for internal absorption of photons. Previously, on older generation PET scanners attenuation correction of emission images was performed by obtaining a transmission scan utilizing a 511 keV source (such as 68Ge) or 137Cs (662 keV). The typical patient dose from a transmission scan using a 68Ge source was about 0.08-0.13 mSv [51]. With the advent of PET/CT, x-ray transmission images from a CT scan are now used for attenuation correction.
Transmission scans using a radionuclide source use the PET detectors for data collection and are acquired over many breathing cycles (standard transmission scans require 18 to 35 minutes to complete) [7,16,41]. Standard transmission scans contain high levels of noise and have low resolution compared to CT images [41]. The quality of attenuation corrected images will be degraded by patient motion between the emission and transmission acquisitions. Motion will create image artifacts- either photopenic defects or apparent lesions. Artificial lesions created by motion between the two data sets will not be present on non-attenuated corrected images.
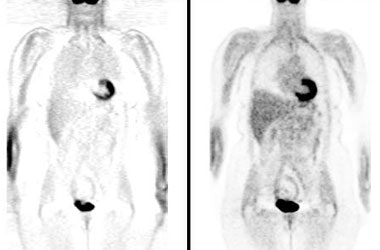
Attenuation correction: The images below are from the same patient. The image on the left is non-attenuation corrected (the lungs and skin surface appear warm). The right image is corrected for attenuation. Note the improved visualization of structures deep within the body. |
|
|
CT scanners are now incorporated into PET scanners for the purpose of co-registration imaging and attenuation correction- see discussion below.
PET/CT and Co-registration Imaging:
Co-registration of the PET and CT data is a benefit of combined PET/CT units. Combined PET-CT images are more effective than PET images alone in precisely localizing neoplastic lesions and in distinguishing normal uptake from juxtaposed neoplastic lesions [20,23,32,47]. PET-CT fusion data can lead to a significant change in impression in 20% of oncologic cases [15]. Even in cases where no change in impression occurred, there is greater diagnostic confidence [15], improved diagnostic accuracy [47], and fewer equivocal lesions [32]. PET-CT may affect patient treatment significantly and improve specificity more than sensitivity [15]. The results of PET/CT exams can impact on patient management in 14-18% of patients [20,32]. Settings in which PET/CT imaging is particularly useful include radiation therapy planning, preoperative surgery/biopsy planning, in the evaluation of head and neck tumors, and in the detection of recurrent abdominal and pelvic malignancies [39,47].
The CT examination can also be used for attenuation correction. The advantages of using CT is that the transmission scan be be acquired in under one minute (thereby shortening the overall PET exam time by 25-30% when compared to a standard PET imaging [56]), the images have higher spatial resolution, and there is significantly improved lesion classification because of the ability to co-register the PET and CT data sets [7,10]. An additional benefit is that by eliminating the radionuclide source for transmission scanning, CT units remove the need for periodic replacement of decayed transmission sources [7].
The CT examination can be a diagnostic examination if the patient has no prior imaging studies, or a low dose exam performed for attenuation and localization purposes. In general, for a localizer exam a 40-80 mA tube current and 120-140 kVp x-ray energy is used [28,48]. The tube current can be adjusted down to 40 or 60 mA in very small patients, or up to 120-160 mA in larger patients [28]. Before the exam, patients should remove all metal that could lead to streak artifacts on the CT transmission scan [26]. Having the patient's arms by their sides also produces beam hardening artifact and therefore, whenever possible, the patients arms should be immobilized above their head [28]. For head and neck cancer patients, a second PET/CT acquisition can be performed with the arms down to better image the neck region [68]. Imaging though the chest is optimally performed during a normal expiration breath hold [28]. If the patient cannot maintain an expiration breath hold for the CT exam, then the study should be performed with the patient breathing quietly [28].
For a high-quality PET/CT examination (PET and diagnostic CT exam) patient radiation exposure is increased compared to a standard PET examination (about 25 mSv [2.5 rad] compared to about 7 mSv [0.7 rad]) [44,66]. The radiation dose to the patient can be lowered if the CT examination is performed using a low dose technique (which would decrease the effective dose from the CT portion of the examination to less than 5 mSv (0.5 rad)) [44]. However, even at low tube currents the radiation exposure from CT can be 1 to 2 orders of magnitude larger than the small dose from a 68Ge source (tens of miliirads at most) [71]. For perspective- background radiation exposure in the United States is about 360 mrem (3.6 mSv) per year [66]. The estimated dose for a round-trip transatlantic flight between New York and Paris is about 12 mrems (0.12 mSv) [66].
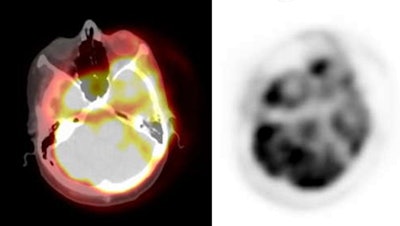
PET/CT exam: The images below are from a PET/CT exam. The patient moved between the acquisition of the CT study and the PET exam- hence- there is misalignment of the two exams. |
|
|
When using CT for attenuation correction- attenuation values are energy dependent [24]. Attenuation maps are displayed in HU's and the measured coefficients at the original acquisition energy are scaled on a pixel-by-pixel basis to 511 keV [24]. The scaled CT images are also interpolated from the CT to the PET spatial resolution [70].
Most PET/CT scans are acquired using a combined PET/CT unit. However, software fusion can be applied to PET and CT data sets acquired on separate units. Studies have shown that thoracic alignment does no significantly differ between PET/CT or from separate PET and CT exams obtained on the same day when carefully matched for anatomic positioning and respiration [54]. If the PET and CT exams are acquired on separate units, the use of a vacuum cushion aids in reproducible patient positioning which improves fusion [46].
Although the CT exam used for attenuation correction is generally not a diagnostic quality study, the CT examination can reveal clinically important findings that are not FDG avid [61]. Major non-FDG avid clinical findings have been described in up to 4% of patients and these findings can affect clinical management [61]. Therefore, review of the CT examination is important and should be included in the final report [61]. Missed findings on the CT scan can be related to expiratory imaging of the lungs (or respiratory motion), lack of IV contrast, beam hardening artifacts if the patient has their arms by their sides, lack of oral contrast, and low mA resulting in image noise [73]. Small lung nodules are commonly not identified on expiratory or motion degraded images. However, small nodules (less than 1cm) can be identified on the CT images and lung windows should always be reviewed. In patients with a known oncologic history, lack of FDG accumulation in these small nodules does not exclude malignancy [64]. Almost 20% of such nodules will subsequently prove to be malignant and the risk is increased if there is more than one nodule [64]. [73].
Image artifacts on PET/CT exams:
Respiratory motion: Respiration during image acquisition was not a significant problem with older PET scanners that acquired 68Ge emission scans for attenuation correction. Both the emission and transmission data sets were equally blurred by this motion [71]. One problem with CT attenuation correction is that the emission PET exam is acquired over several minutes while the CT exam is acquired in a single breath hold. Co-registration of the PET and CT data sets is not always perfect due to respiratory motion- this problem is most significant in the lung bases, lung periphery, and liver dome [3,10,12,13]. A curvilinear cold artifact paralleling the dome of the diaphragm at the lung bases is a frequently noted artifact on PET/CT image acquisitions obtained at free tidal breathing (it can be seen in up to 84% of cases [39]) [13,50]. The artifact is usually mild, but can be significant if the CT exam is acquired with deep inspiration [16]. The artifact is due to an inaccurate correction for photon attenuation at the lung base because of inconsistencies in the liver-lung interface between the PET and CT acquisitions [13]. This artifact can also result in a significant decrease in measured SUV within a pulmonary lesion in the lung base [16,50]. A mismatch between the data sets can also lead to the erroneous placement of a lung lesion into adjacent structures such as the liver or a rib [12]. Liver lesions may be incorrectly localized to the lung bases [13]. Shallow breathing during the CT exam can help to decrease the artifact and improve fusion of the data sets [62], however, CT images acquired with shallow respiration have been shown to result in the failure to identify small lung nodules compared to breath hold imaging [58]. The artifact is best minimized when the CT examination is acquired with a normal expiration breath hold protocol [16]. CT images acquired during normal expiration rather than with shallow respiration demonstrate improved co-registration of pulmonary nodules with PET images [12]. Alternatively, respiration-averaged CT has also been shown to reduce misalignment between the CT and PET data sets [50]. All of these techniques will likely result in the inability to confidently identify all small lung nodules and the chest CT portion of the exam should be regarded as not of diagnostic quality if not performed with breath hold at end-inspiration [58,62]. Some centers will perform an additional low dose CT scan of the chest during maximal inspiration for better identification of lung nodules [67].
Significant registration errors can also involve the liver due to respiration- particularly the diaphragmatic area [72].
Reconstruction artifacts can also occur in the pelvis due to displacement of organs and bowel by bladder or rectal filling [25,30]. This problem is best solved by keeping the interval between the CT scan and the PET scan as short as possible [25] and by beginning the PET scan over the pelvis and proximal thighs [68].
Another artifact is related to truncation which occurs due to a discrepancy in the size of the field of view (FOV) for the CT scanner versus the PET scanner [60]. When large patients undergo CT imaging portions of their body may lie outside the FOV resulting in truncation artifact which appears as poorly defined areas of high density along the margins of the patient [60]. Because the PET scanner has a larger field of view- the entire patient is imaged. The discrepancy between the field of views causes some sections of the PET emission data not to have any corresponding attenuation correction factors [60]. The net effect is an overestimation of the activity corresponding to the image rim (the ill-defined area of high density at the margin of the CT scan) and an underestimation in the region without correction factors (the portion of the patient that was not included on the CT image) [60]. SUV measurements in these areas will be incorrect [60].
Calculated SUV's may be slightly higher when using CT attenuation correction and caution should be exercised if comparing data between CT and separate transmission scans [7].
It should be remembered that the use of CT for attenuation correction does result in additional patient radiation exposure [25].
Effects of contrast and high attenuation objects:
Oral and IV contrast, as well as pacemakers and metallic implants can result in image artifacts. CT based attenuation correction is subject to error because it requires conversion of the x-ray attenuation factors obtained at CT (120-140 kVp) to attenuation at PET energy (511 keV) [68]. On previous attenuation correction algorithms, contrast would be misclassified as high-density bone and the 511-keV-equivalent attenuation coefficient values for the contrast are over-estimated resulting in over-correction for attenuation in PET images [22]. This produces an apparent increased concentration of radiotracer in the contrast region on the attenuated corrected images [22]. Newer attenuation correction algorithms have reduced or eliminated the problem of artifactually increased activity in regions of high contrast enhancement [68].
IV contrast and PET/CT imaging: IV contrast enhanced CT exams may result in PET image artifacts if used for attenuation correction [8,19]. The artifact appears to be related to the transient bolus of undiluted contrast within the large veins of the chest and often produces a focal area of increased activity on attenuation corrected PET images (i.e.: an overestimation of FDG activity) [8,19,27]. This is because the contrast agent results in greater attenuation of CT photons, compared to PET photons which results in an overestimation of PET activity on reconstructed images [19,27]. The effect is most pronounced at the location of the CT contrast agent, but does also affect other regions within the slice [17]. At a contrast concentration of 200 HU, the percent overestimation of FDG activity was between 10% to 15%, but it can be as high as 45% at an HU of 1,360 [19]. Organs that demonstrate strong contrast enhancement, such as the kidneys and liver, can also be affected [19]. Generally, the SUV is elevated between 5% to 7% [36], however, overestimation of activity can be as high as 25% in the kidneys, and between 12-15% in the liver [19]. In patients with liver tumors, it is possible that over-estimation of background hepatic activity could result in obscuration of subtle hepatic lesions [19,36]. Although a similar effect is seen in the kidneys, the generally high concentration of activity in the kidney, ureters, and urinary bladder make evaluation of these structures more difficult anyway [19]. The effects of contrast agents could be avoided by performing the fusion/attenuation correction CT exam without contrast, and then performing a contrast enhanced exam after completion of the PET study [19]. Unfortunately, this results in increased patient radiation exposure [19]. Other articles suggest that although there is slight artificial elevation in SUV values following I.V. contrast enhanced PET/CT imaging, it is not clinically significant and does not alter exam interpretation [36,56]. A dual-phase contrast injection (80 and 60 mL at 3 and 1.5 mL/sec respectively) in the craniocaudal direction with a 50 second delay has been proposed as a CT protocol which yields consistent high image quality with few artifacts [37]. Alternatively, caudocranial scanning or using a saline chaser immediately after contrast injection can also aid in avoiding contrast associated artifacts [67].
Oral contrast and PET/CT Imaging: High density barium will attenuate more CT x-rays with energies of 70-140 keV, than 511 keV photons due to the high atomic number of barium which results in an increased fraction of photoelectric interactions- this produces an increase in Hounsfield units on CT images [22]. An over-estimation in PET activity of up to 20% can be seen in regions where barium-based oral contrast is present [65]. When a lesion is present within the contrast volume, this can obscure the lesion [22]. Also- high density oral contrast can produce beam hardening artifacts which can produce apparent areas of increased FDG activity on attenuation corrected images [9,14,22]. Oral contrast with 1.3% barium, as used commonly in CT contrast studies, does not appear to be associated with the creation of attenuation correction artifacts [14]. However, if the concentration of the oral contrast in the lumen increases markedly as a result of significant water resorption, it is possible that artifacts may be produced [14]. Oral contrast may also affect SUV's generated for intralumenal lesions resulting in an over-estimation of the lesion's SUV [22], however, this effect does not appear to be medically significant [17].
A way to avoid contrast-induced artifacts is the use of a water as an oral contrast agent, unfortunately, this may result in increased urinary urgency during the scan [27,30]. Another option is to use VoLumen - a low density barium sulfate suspension that acts as a negative oral contrast agent [65,68]. Substances can be added to avoid absorption of the water- for instance, a combination of water, 2.5% mannitol (to increased bowel distention due to osmotic properties), and 0.2% locust beam gum (LBG- to avoid absorption due to a gelling action) [27,30]. Superior bowel distention can be achieved with this preparation because mannitol enhances secretion of water into the bowel, and LBG prevents the intestinal absorption of water [30]. The cost of this preparation is about $3 for one liter (which is close to the price of a 500 ml bottle of barium contrast- about $3.50) [30]. A small number of patients may experience watery diarrhea after the exam from this preparation [30].
Metallic hardware: Metal implants associated with chemotherapy ports, artificial joints, or dental fillings cause beam hardening and scatter artifacts which can produce apparent increased tracer activity on the corrected PET images [26]. Most metals exhibit strong photoelectric absorption of x-rays, but interact with 511 keV gamma rays primarily via Compton scattering [38]. The CT attenuation correction scaling algorithm does not account for this effect and causes overcorrection of the PET images [38]. Overcorrection is not a signifnicant problem with conventional PET scanners that use 68Ge/68Ga or 137Cs transmission sources with gamma energies of 511 keV and 662 keV, respectively [38].
Cardiac pacers and central venous catheter reservoirs can produce a focal area of artifactual increased FDG uptake on CT corrected PET images [29]. For cardiac PET imaging, defibrillator leads can be particularly problematic if they are placed in close relationship to the left ventricle [38]. Falsely elevated FDG uptake of 44-81% be seen at the lead location [38]. This focal area of increased activity can mask a perfusion defect and can also interfere with image normalization [38]. Reservoirs usually produce only a mild focus of increased activity, while pacemakers produce a more moderate focal abnormality [29].
Dental hardware can also produce beam hardening artifacts which can produce apparent areas of increased FDG activity on attenuation corrected images [9,14].
Review of emission images will not demonstrate the artifacts if they are related to beam hardening [9].
PET/MRI:
Photomultiplier tubes used in standard PET detectors are sensitive to magnetic fields which will cause electrons to deviate from their original trajectory and result in loss of gain [59]. One way to overcome this problem would be to use optical fibers to carry the scintillation light outside the fringe of the magnetic field, but the use of such long fibers will result in light loss leading to degradation of energy and timing resolution [59]. Avalanche photodiodes (APD) are silicon-based devices that can replace photomultiplier tubes permitting fusion of PET and MRI data [59]. APD's do not show degradation even in magnetic fields up to 9.4T [59].
Advantages of PET- Quantification
PET provides quantitative in-vivo measurement of functional processes: perfusion, metabolism, and receptors. PET can localize and measure the distribution of neuroreceptors by detecting sub-nanomolar concentrations of labeled drugs. The spatial resolution of PET is superior to SPECT (5 -8 mm) and it has accurate correction for photon attenuation which is not yet available for SPECT imaging. The high temporal resolution of PET (high count rate) also permits dynamic imaging.
Absolute quantification of data is performed using compartmental kinetic models [42]. The dynamic behavior of each tracer in vivo is assumed to follow a standard three-compartment kinetic model with two tissue compartments (an extravascular pool of tracer in the tissue and a bound or non-displaceable component) and a single arterial input function (arterial concentration of free, unmetabolized tracer) [42]. Unfortunately, obtaining arterial blood samples is difficult [42].
PET agents and their uses (Table)
| Agent | Images |
| F-18 fluorodeoxyglucose | Regional glucose metabolism |
| F-18-fluorodeoxythymidine | Tumor cell proliferation |
| 18-F-fluoro-L-tyrosine | Tumor protein synthesis |
| 18-F-fluoro-DOPA | Neuroendocrine tumors, CNS tumors, Parkinsons disease |
| 18-F-fluoromisonidazole | Tumor hypoxia |
F-18 sodium fluoride |
Bone
tumors |
C-11
methionine |
Amino acid uptake/protein
synthesis |
| C-11 choline | Cell membrane proliferation |
| C-11 deoxyglucose | Regional brain metabolism |
| O-15 oxygen | Metabolic rate of oxygen utilization/OEF |
| C-11 carbon monoxide | Cerebral blood volume |
| O-15 carbon monoxide | Cerebral blood volume |
| O-15 water | Cerebral blood flow |
| O-15 carbon dioxide (Inhaled) | Cerebral blood flow |
| C-11 butanol | Cerebral blood flow |
| C-11 N-methylspiperone | Dopamine D2 and Serotonin S2 receptors |
| F-18 N-methylspiperone | D2 and S2 receptors |
| C-11 raclopride | D2 receptors |
| F-18 spiperone | D2 receptors |
| Br-76 bromospiperone | D2 receptors |
| C-11 carfentanil | Opiate mu receptors |
| C-11 flumazenil | Benzodiazepine (GABA) receptors |
PET Agent Dose Estimates:
18F-FDG:
18F is produced in a cyclotron through proton bombardment of enriched 18O-water [6]. 18F decays to stable 18O by positron emission with a half life of 109.77 minutes. A standard PET emission scan delivers a dose of 5-10 mSv (0.5 to 1 rad) to the body (1 mSv = 0.1 rad) [25,44]. A traditional germanium-based transmission scan delivers no significant additional dose to the patient [25]. A high-quality PET/CT examination delivers an effective dose of about 25 mSv (2.5 rad) [44]. The radiation dose to the patient can be lowered if the CT examination is performed using a low dose technique (which would decrease the effective dose from the CT examination to less than 5 mSv (0.5 rad) [44]. The urinary bladder wall receives the largest dose, but the dose is variable and affected by bladder volume and frequency of voiding [6]. In infants, the bladder dose is about 4 fold higher than adults and absorbed dose to the brain is about 10-fold higher [125]. Estimated absorbed doses are listed below [6]:
| Target organ | Absorbed dose in rad/mCi (mean +/- SD) |
| Brain | 0.17 +/- 0.044 |
| **Heart wall | 0.25 +/- 0.13 |
| Kidneys | 0.078 +/- 0.022 |
| Liver | 0.088 +/- 0.031 |
| Lungs | 0.056 +/- 0.031 |
| Pancreas | 0.052 +/- 0.006 |
| Red marrow | 0.040 +/- 0.006 |
| Spleen | 0.056 +/-0.008 |
| **Urinary bladder wall | 0.27 +/- 0.16 |
| Ovaries | 0.041 +/- 0.005 |
| Testes | 0.041 +/- 0.005 |
| Whole body | 0.043 +/- 0.002 |
18F-FDG in pregnancy:
The radiation dose estimates are 2.2 x 10-2 mGy/MBq in early pregnancy, 2.2 x 10-2 mGy/MBq at 3 months gestation, 1.7 x 10-2 mGy/MBq at 6 months gestation, and 1.7 x 10-2 mGy/MBq at 9 months gestation [33].
REFERENCES:
(1) J Nucl Med 2001; Votaw JR, White M. Comparison of 2-dimensional and 3-dimensional cardiac 82Rb PET studies. 42: 701-706
(2) J Nucl Med 2000; Melcher CL. Scintillation crystals for PET. 41: 1051-1055
(3) Radiol Clin N Am 2001; Fahey FH. Positron emission tomography instrumentation. 39: 919-929
(4) J Nucl Med 2001; Adam LE, et al. Performance of a whole-body PET scanner using curve-plate NaI(Tl) detectors. 42: 1821-1830
(5) J Nucl Med 2001; Knesaurek K. New developments in PET instrumentation: quo vadis PET. 1831-1832
(6) J Nucl. Med 2002; Hays MT, et al. MIRD Dose Estimate Report No. 19: Radiation Absorbed Dose Estimates from 18F-FDG. 43: 210-214
(7) J Nucl Med 2002; Nakamoto Y, et al. PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation-corrected images. 43: 1137-1143
(8) J Nucl Med 2002; Antoch G, et al. Focal tracer uptake: a potential artifact in contrast-enhanced dual-modality PET/CT scans. 43: 1339-1342
(9) AJR 2002; Goerres GW, et al. Positron emission tomography and PET CT of the head and neck: FDG uptake in normal anatomy, in benign lesions, and in changes resulting from treatment. 179: 1337-1343
(10) Radiology 2002; Hany TF, et al. PET diagnostic accuracy: improvement with in-line PET-CT systems: initial results. 225: 575-581
(11) Ann Thorac Surg 1998; Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. 65: 1821-29
(12) J Nucl Med 2002; Goerres GW, et al. Accuracy of image coregistration of pulmonary lesions in patients with non-small cell lung cancer using an integrated PET/CT system. 43: 1469-1475
(13) J Nucl Med 2003; Osman MM, et al. Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. 44: 240-243
(14) J Nucl Med 2003; Cohade C, et al. Initial experience with oral contrast in PET/CT: phantom studies and clinical studies. 44: 412-416
(15) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(16) Radiology 2003; Goerres GW, et al. Respiration-induced attenuation artifact at PET/CT: technical considerations. 226: 906-910
(17) J Nucl Med 2003; Dizendorf E, et al. Cause and magnitude of the error induced by oral CT contrast agent in CT based attenuation correction of PET emission studies. 44: 732-738
(18) J Nucl Med 2003; Tarantola G, et al. PET instrumentation and reconstruction algorithms in whole-body applications. 44: 756-769
(19) Radiology 2003; Nakamoto Y, et al. Effects of nonionic intravenous contrast agents at PET/CT imaging: phantom and canine studies. 227: 817-924
(20) J Nucl Med 2003; Bar-Shalom R, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. 44: 1200-1209
(21) J Nucl Med 2003; Karp JS, et al. Performance of a brain PET camera based on anger-logic gadolinium oxyorthosilicate detectors. 44: 1340-1349
(22) J Nucl Med 2003; Nehmeh SA, et al. Correction for oral contrast artifacts in CT attenuation-corrected PET images obtained by combined PET/CT. 44: 1940-1944
(23) J Nucl Med 2004; Czernin J, Schelbert H. PET/CT: facts, opinions, hopes, and questions. 45 (Suppl): 1S-3S
(24) J Nucl Med 2004; Townsend DW, et al. PET/CT today and tomorrow. 45 (Suppl): 4S-14S
(25) J Nucl Med 2004; Vogel WV, et al. PET/CT: panacea, redundancy, or something in between. 45 (Suppl): 15S-24S
(26) J Nucl Med 2004; Beyer T, et al. Acquisition protocol considerations for combined PET/CT imaging. 45 (Suppl): 25S-35S
(27) J Nucl Med 2004; Antoch G, et al. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual modality 18F-FDG PET/CT. 45 (Suppl): 56S-65S
(28) J Nucl Med 2004; Wahl RL. Why nearly all PET of abdominal and pelvic cancers will be performed as PET/CT. 45 (Suppl): 82S-95S
(29) J Nucl Med 2004; Halpern BS, et al. Cardiac pacemakers and central venous lines can induce focal artifacts on CT-corrected PET images. 45: 290-293
(30) Radiology 2004; Antoch G, et al. Dual-modality PET/CT scanning with negative oral contrast agent to avoid artifacts: introduction and evaluation. 230: 879-885
(31) Radiographics 2004; Kapoor V, et al. An introduction to PET-CT imaging. 24: 523-543
(32)
(33) J Nucl Med 2004; Stabin MG. Proposed addendum to previously published fetal dose estimate tables for 18F-FDG. 45: 634-635
(34) J Nucl Med 2004; Lartizien C, et al. A lesion detection observer study comparing 2-dimensional versus fully 3-dimensional whole-body PET imaging protocols. 45: 714-723
(35) J Nucl Med 2004; Halpern BS, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. 45: 797-801
(36) J Nucl Med 2005; Yau YY, et al. Application of intravenous contrast in PET/CT: does it really introduce signifncant attenuation correction error? 46: 283-291
(37) J Nucl Med 2005; Beyer T, et al. Optimized intraveous contrast administration for diagnostic whole-body 18F-FDG PET/CT. 46: 429-435
(38) J Nucl Med 2005; DiFilippo FP, Brunken RC. Do implanted pacemaker leads and ICD leads cause metal-related artifact in cardiac PET/CT? 46: 436-443
(39) Radiol Clin N Am 2004; Alavi A, et al. PET: a revolution in medical imaging. 42: 983-1001
(40) Radiol Clin N Am 2004; Surti S, et al. PET instrumentation. 42: 1003-1016
(41) Radiol Clin N Am 2004; Alessio AM, et al. PET/CT scanner instrumentation, challenges, and solutions. 42: 1017-1032
(42) Radiol Clin N Am 2004; Acton PD, et al. Quantification in PET. 42: 1055-1062
(43) J Nucl Med 2005; Halpern BS, et al. Optimizing imaging protocols for overweight and obese patients: a lutetium orthosilicate PET/CT study. 46: 603-607
(44) J Nucl Med 2005; Brix G, et al. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. 46: 608-613
(45) J Nucl Med 2005; Brasse D, et al. Correction methods for random coincidences in fully 3D whole-body PET: impact on data and image quality. 46: 859-867
(46) AJR 2005; Nakamoto Y, et al. Accuracy of image fusion using a fixation device for whole-body cancer imaging. 184: 1960-1966
(47) J Nucl Med 2005; Coleman RE, et al. Concurrent PET/CT with an integrated imaging system: intersociety dialogue from the joint working group of the american college of radiology, the society of nuclear medicine, and the society of computed body tomography and magnetic resonance. 46: 1225-1239
(48) J Nucl Med 2005; Ford H. CT in PET/CT: essential features of interpretation. 46: 1249-1251
(49) J Nucl Med 2005; Osman MM, et al. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. 46: 1352-1355
(50) J Nucl Med 2005; Pan T, et al. Attenuation correction of PET images with respiration-averaged C images in PET/CT. 46: 1481-1487
(51) J Nucl Med 2005; Schwaiger M, et al. PET/CT: Challenge for nuclear cardiology. 46: 1664-1678
(52) Radiol Clin N Am 2005; Jadvar H, et al. PET in pediatric diseases. 43: 135-152
(53) J Nucl Med 2005; Watson CC, et al. Optimizing injected dose in clinical PET by accurately modeling the counting-rate response functions specific to individual patient scans. 46: 1825-1834
(54) Radiology 2005; Krishnasetty V, et al. Comparison of alignment of computer-registered data sets: combined PET/CT versus independent PET and CT of the thorax. 237: 635-639
(55) J Nucl Med 2006; Lodge MA, et al. Comparison of 2-dimensional and 3-dimensional acquisition for 18F-FDG PET oncology studies performed using an LSO-based scanner. 47: 23-31
(56) AJR 2006; Mawlawi O, et al. Quantifying the effect of IV contrast media on integrated PET/CT: clinical evaluation. 186: 308-319
(57) Radiology 2006; von Schulthess GK, et al. Integretated PET/CT: current applications and future directions. 238: 405-422
(58) J Nucl Med 2006; Allen-Auerbach M, et al. Standard PET/CT of the chest during shallow breathing is inadequate for comprehensive staging of lung cancer. 47: 298-301
(59) J Nucl Med 2006; Pichler B, et al. Peformance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. 47: 639-647
(60) AJR 2006; Mawlawi O, et al. Truncation artifact on PET/CT: impact on measurements of activity concentration and assessment of a correction algorithm. 186: 1458-1467
(61) AJR 2006; Bruzzi JF, et al. Incidental findings on integrated PET/CT that do not accumulate 18F-FDG. 187: 1116-1123
(62) AJR 2006; Gilman MD, et al. Optimal CT breathing protocol for combined thoracic PET/CT. 187: 1357-1360
(63) J Nucl Med 2006; Kemp BJ, et al. NEMA NU 2-2001 performance measurements of an LYSO-based PET/CT system in 2D and 3D acquisition modes. 47: 1960-1967
(64) J Nucl Med 2007; O JH, et al. Clinical significance of small pulmonary nodules with little or no 18F-FDG uptake on PET/CT images of patients with nonthoracic malignancies. 48: 15-21
(65) Radiographics 2007; Prabhakar HB, et al. Bowel hot spots at PET-CT. 27: 145-159
(66) J Nucl Med 2007; Schoder H, Gonen M. Screening for cancer with PET and PET/CT: potential limitations. 48: 4S-18S
(67) J Nucl Med 2007; Kuehl H, et al. Can PET/CT replace separate diagnostic CT for cancer imaging? Optimizing CT protocols for imaging cancers of the chest and abdomen. 48: 45S-57S
(68) AJR 2007; Wong TZ, et al. Practical approach to diagnostic CT combined with PET. 188: 622-629
(69) J Nucl Med 2007; Surti S, et al. Performance of Phillips Gemini TF PET/CT scanner with special considerations for its time-of-flight imaging capabilities. 48: 471-480
(70) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-385
(71) J Nucl Med 2007; Bacharach SL. PET/CT attenuation correction: breathing lessons. 48: 677-679
(72) J Nucl Med 2007; Vogel WV, et al. Evaluation of image registration in PET/CT of the liver and recommendations for optimized imaging. 48: 910-919
(73) J Nucl Med 2007; Gollub MJ, et al. Limitations of CT during PET/CT. 48: 1583-1591