Software developer Olea Medical has received U.S. Food and Drug Administration (FDA) clearance for its Olea Sphere 3.0 medical imaging enterprise software.
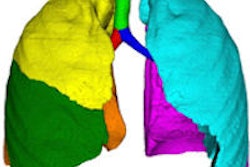
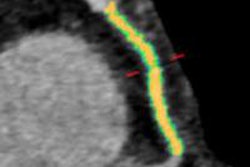
Olea Sphere is for image archiving, postprocessing, and communication. It helps standardize both viewing and analysis capabilities of functional and dynamic imaging datasets acquired with MRI and CT from different vendors, the firm said.
Olea Sphere is compliant with the DICOM standard and Windows or Linux operating systems. It runs on any standard off-the-shelf workstation or it can be used through thin deployment. Olea Sphere maintains the traceability of patient data through an automatic logout mode.
New postprocessing modules to be introduced in the U.S. following the FDA clearance are Intravoxel Incoherent Motion, Metabolic, and Relaxometry.