(Booth 9157) French computer-aided detection (CAD) software developer Median Technologies of Sophia Antipolis will demonstrate its progress in commercializing its Lesion Management Solution (LMS) line of software.
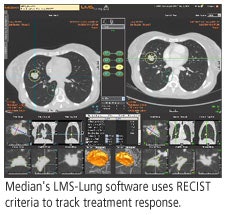
Median said it has launched the product line in Europe and opened a sales office in Minneapolis to direct U.S. activities. It is also pursuing partnerships with academic centers to foster cooperation between radiologists and oncologists.
Median said that the LMS software is designed to automate the tasks that radiologists normally carry out manually. LMS applications generate reports according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria used to evaluate the treatment response of solid tumors. The software can also be integrated with PACS or RIS networks.
Median is also developing LMS-Colon, which does not yet have FDA clearance and will be shown as a work-in-progress for CT colonography applications.