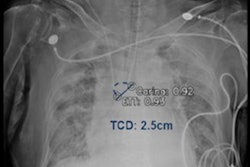
The U.S. Food and Drug Administration (FDA) has cleared GE Healthcare's artificial intelligence (AI) software for assessing endotracheal tube placement on x-ray images.
GE's Critical Care Suite 2.0 software features algorithms that help radiologists triage cases and reduce review time, according to the firm. It is loaded on a mobile x-ray system and detects endotracheal tubes on chest x-rays, providing an assessment of tube positioning. Its results are then sent immediately to the radiologist.
GE has been distributing Critical Care Suite 2.0 under the FDA's COVID-19 imaging guidance since November 2020. This new clearance will allow it to be used outside of a public health emergency, GE said.