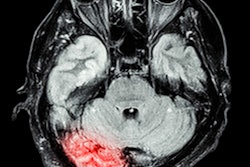
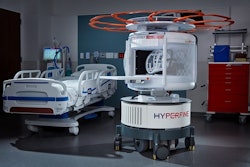
The U.S. Food and Drug Administration (FDA) has cleared an update of Hyperfine's AI-powered software for its Swoop portable brain MRI system
The software upgrade offers improved image quality for Swoop's diffusion-weighted imaging (DWI) sequence by expanding its denoising capability, the company said.
The new software will become available over the next few months, according to Hyperfine.