As the prostate cancer treatment paradigm shifts from traditional chemotherapy to cutting-edge theranostics, the tasks of identifying whether cancer is significant and if and where it has spread are still difficult.
However, recent prostate cancer AI research initiatives clearly demonstrate that decision-support algorithms can facilitate improved diagnostic performance and better-targeted biopsies.
In this AuntMinnie.com special article, we explore hurdles to the use of AI in prostate MRI.
As the prostate cancer treatment paradigm shifts from traditional chemotherapy to cutting-edge theranostics, the tasks of identifying whether cancer is significant and if and where it has spread are still difficult.
However, recent prostate cancer AI research initiatives clearly demonstrate that decision-support algorithms can facilitate improved diagnostic performance and better-targeted biopsies.
In this AuntMinnie.com special article, we explore hurdles to the use of AI in prostate MRI.
Prostate cancer is the second most common cancer in men around the world. The American Cancer Society anticipates nearly 300,000 new U.S. cases in 2024.
Looking forward, The Lancet Commission on prostate cancer projected trends in the incidence of prostate cancer and related mortality in the next 10-15 years as only rising (from 1.4 million in 2020 to 2.9 million by 2040). Although age-adjusted mortality from prostate cancer is falling in high-income countries, it is rising in lower- and middle-income countries, the commission noted.
Long-standing challenges
As many radiologists and urologists know, the problem is that despite the volume of cases in the records, clinically significant prostate cancer (csPCa) forms are still not easy to distinguish. This is because prostate cancer can present from a clinically irrelevant or indolent form to virulent, aggressive, and metastatic, and MRI presents an unclear picture. Thus, researchers have been assessing various AI strategies.
As an example of the speed at which medical AI can develop, the Prostate Cancer-Imaging AI (PI-CAI) Challenge brought together over 200 teams and 62 radiologists from 20 countries to address some of the long-standing challenges of clinical prostate cancer. Radboud University Medical Center in the Netherlands coordinated the project, using 10,207 MRI examinations performed from January 1, 2012 to December 31, 2021.
For PI-CAI, teams trained and validated modern AI algorithms and estimated radiologists’ performance with clinically significant prostate cancer detection and diagnosis. The findings were published in The Lancet Oncology's July edition.
“AI detects nearly seven percent more significant prostate cancers than the group of radiologists,” Radboud researchers wrote in their overview of PI-CAI's outcomes. “Additionally, AI identifies suspicious areas, later found not to be cancer, fifty percent less often. This means the number of biopsies could be halved with the use of AI.”
However, the algorithms lack prospective validation, which is a requirement to test clinical applicability. Consequently, the PI-CAI algorithms are currently not available for patients in clinical settings.
Medical functions diverse
Baris Turkbey, MD, a prostate cancer imaging specialist at the U.S. National Institutes of Health (NIH), characterized the focus of AI research in prostate MRI as “quite diverse, incorporating image quality, organ segmentation, intraprostatic lesion detection, and [Prostate Imaging Reporting & Data System] PI-RADS classification.”
 Baris Turkbey, MD.
Baris Turkbey, MD.
Turkbey has been with the Nation Cancer Institute's (NCI) molecular imaging branch since 2007. Turkbey researches prostate cancer imaging using multiparametric MRI, PET/CT, as well as image-guided biopsy and treatment techniques -- and AI. He's also a member of the PI-RADS Steering Committee.
The PI-CAI challenge, Turkbey said, provides level 2b evidence that a prostate MRI AI model could substantially reduce overdiagnosis and potentially eliminate unnecessary biopsies in a primary diagnostic setting, achieving 50.4% fewer false positives and detecting 20% fewer indolent cancers than a cohort of 62 radiologists. Turkbey assessed PI-CAI for a September 11 article in Nature Review Urology.
“The study by the PI-CAI group is an important step toward documenting the expected standards for AI research in diagnostic radiology,” Turkbey wrote. “This study, along with similar future work, not only will support many institutions and researchers in the field but also will guide us toward a more robust and confident implementation of AI in our practice.”
The PI-CAI Challenge was a large observational study. It involved multiple sites (9,207 cases from 11 sites in the Netherlands for training and tuning, and 1,000 examinations in the Netherlands and Norway for testing) and various commercial 1.5-tesla or 3-tesla scanners.
While the results were encouraging, PI-CAI's approach noted several limitations. Importantly, the study did not allow for the inclusion of low-quality MRIs, which are commonly encountered and "unfortunately" used in real-life prostate MRI practice, Turkbey noted. Also, in real-life settings, AI models would be used with an expert involved, but the use of AI as an assistive tool during prostate MRI readouts was not investigated in PI-CAI.
Most evolved
There are currently three main categories of AI for prostate MRI, Turkbey explained to AuntMinnie.com.
- Algorithms that evaluate the quality of MRI images
- Prostate segmentation algorithms
- Software that finds and classifies lesions
"Finding lesions and classifying them is the most difficult part of prostate imaging," Turkbey said. "Algorithms that deal with prostate segmentation are the most evolved.“
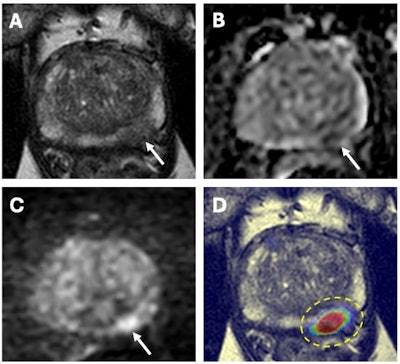 67-year-old male with a serum PSA of 6.5ng.ml. Axial T2W MRI (A) shows a hypointense lesion in the left apical-mid peripheral zone (arrow) which demonstrates focal diffusion restriction on ADC map (B) and high b-value diffusion-weighted MRI (C) (arrows). AI output of a biparametric prostate MRI lesion detection AI model demonstrates high cancer probability within the left apical-mid peripheral zone lesion (dashed yellow circle) (D). MRI/TRUS fusion guided biopsy revealed Gleason 3+4 prostate cancer within this lesion. Images and caption courtesy of the National Cancer Institute.
67-year-old male with a serum PSA of 6.5ng.ml. Axial T2W MRI (A) shows a hypointense lesion in the left apical-mid peripheral zone (arrow) which demonstrates focal diffusion restriction on ADC map (B) and high b-value diffusion-weighted MRI (C) (arrows). AI output of a biparametric prostate MRI lesion detection AI model demonstrates high cancer probability within the left apical-mid peripheral zone lesion (dashed yellow circle) (D). MRI/TRUS fusion guided biopsy revealed Gleason 3+4 prostate cancer within this lesion. Images and caption courtesy of the National Cancer Institute.
Hurdles to go
"While numerous commercially developed algorithms address prostate segmentation and lesion detection and classification, clinical translation is still limited," Turkbey added. However, "in the sequence of events, the three categories are all linked."
Without good-quality imaging, the second and third categories will fail, according to Turkbey.
"The PI-CAI challenge addresses the third area where radiologists have to invest a lot in their education and experience,” he said. “With prostate imaging, evaluation of the transition zone is very prone to false-positive findings. A good AI model should give you a minimum number of lesions [and determine] where there are clinically significant cancers.”
Although cancer detection sensitivity is important when considering the selection of an AI model for your daily practice, the false-positive rate must be minimal, Turkbey said. Further, developers should conduct failure analyses which will help to better understand the limitations and strengths of the developed AI models.
Unproductive biopsies
AI is also showing promise in helping urologists to plan more effective biopsies.
"A substantial number of men undergo unproductive targeted biopsies [TBs] along with potential complications, which need to be [minimized]," explained Karsten Guenzel, MD, from the department of urology at Vivantes Klinikum Am Urban in Berlin, and colleagues from the department of radiology, for an April article in European Urology Focus.
Their prospective observational study conducted in 2022 explored the diagnostic utility of AI-assisted biopsy planning in suspected prostate cancer. Using AI in conjunction with multiparametric MRI in transperineal biopsy planning (TBP), this study comes at a time when urologists may be using prebiopsy MRI and targeted MRI-ultrasound fusion biopsy more frequently.
"The high number of false positives and the qualitative variations in reporting are substantial limitations for urologists in their biopsy decision-making," noted Guenzel and colleagues.
They produced initial evidence demonstrating the performance of transperineal biopsies planned using PI-RADS categorization from radiology reports along with the use of a commercial computer-assisted detection (CAD) software application. Results were compared to biopsies planned using PI-RADS categorization alone.
For the 262 men included in the study, csPCa was detected in 56% (146/262) of patients. CAD-directed transperineal prostate biopsies yielded a sensitivity of 97%, a significant improvement compared with the 92% sensitivity produced from biopsies based only on PI-RADS categorization (p = 0.007), Furthermore, CAD-directed biopsies solely detected csPCa in 10 (4%) of 262 patients and identified additional csPCa lesions in 8% (22/262).
True (or false) positives
The catch was that while the number of targeted lesions increased by 54% (518 vs. 336), the rate of false positives per case also doubled (0.66 vs. 1.39; p = 0.009).
On a lesion-based level, CAD sensitivities for [grade group (GG)] GG ≥ 2 and ≥ 3 cancers were not statistically superior to the original PI-RADS categorization in the original radiology report (both 90% vs. 86%; p = 0.16–0.21). However, significant improvement was shown for GG ≥ 1 cancers (98% vs. 79%; p < 0.001), according to the authors, who added that using the CAD software, specificity decreased significantly (p < 0.001).
On the other hand, CAD-targeted biopsies achieved higher sensitivity in detecting GG ≥ 2 cancers on a patient level than radiologists (97% vs. 92%; p = 0.007; GG ≥ 3: 97% vs. 88%; p = 0.001). The team added that this sensitivity improvement came at the cost of a higher false-positive rate. They also noted that radiologists showed identical performance to radiological reporting in the study.
CAD-assisted biopsy planning strategies increased the false-positive rate significantly, adding to the already existing limitations of insufficient specificity of PI-RADS scoring, according to the authors. However, the software may enable more personalized biopsy planning depending on urological and patient preferences, they said.
Personalized medicine
"In our study, with the aid of the CAD tool, only 1% of significant cancers would have been missed by [targeted biopsies] alone," Guenzel and colleagues noted.
The findings raise the issue of whether the use of a CAD tool could help eliminate the need for nontargeted systematic biopsies because the vast majority of missed lesions are found by CAD software, they added.
"Furthermore, up to nine unproductive biopsy targets were tolerated under the direction of combined PI-RADS and CAD-ROI TBs compared with PI-RADS targets only, for each target that revealed GG 2 cancer," the authors noted.
Overall, the study suggested that CAD-based biopsy planning strategies could work better for cancer-averse patients. However, the authors noted the difference between a urologist highly experienced in reviewing MRI scans making the region of interest selections versus a less experienced urologist. A less-experienced urologist is likely to increase the number of targeted CAD regions of interest and false positives.
Researchers say that more prospective, randomized trials are needed to evaluate CAD and AI-powered tools to identify their precision medicine benefits and drawbacks, as well as facilitate clinical adoption.
