The U.S. Food and Drug Administration (FDA) has just publicly listed 211 AI-enabled medical devices that have received regulatory clearances since September 28, 2024, a July 10 update showed.
Radiology software products comprise about 81% of the full device update, with the QIH product code -- automated radiological image processing software for medical image management and processing systems -- the most popular at 58 clearances since the last update.
The FDA provided "releasable information," such as summaries of safety and effectiveness, but it omitted most of the information submitted in applications, according to its update.
"The list includes AI-enabled medical devices that were identified primarily based on the use of AI-related terms in the summary descriptions of their marketing authorization document and/or the device’s classification," the agency said.
Separately, in March, FDA, Health Canada, and the U.K.'s Medicines and Healthcare products Regulatory Agency (MHRA) jointly identified guiding principles for informing the development of good machine-learning practice. With the July 10 revision, the FDA simplified its device public listings to the broadest category of AI alone, a change from its established AI/machine-learning (ML) approach.
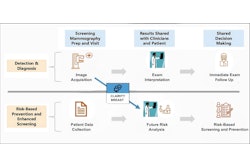
A full radiology breakout reflected one de novo authorization for Clairity's Allix5, dated May 30, 2025. The software-only device (FDA product code SEZ) analyzes images using machine learning, AI, or other image analysis algorithms to produce a numeric probability and/or risk category to predict future breast cancer risk. It is not intended to diagnose, detect, or inform the treatment of cancer, or to guide interpretation of imaging exams, according to FDA public information.
FDA's July 10 update also included the following (FDA codes in parentheses):
- Eight radiological image processing software for radiation therapy (QKB) products.
- Eight medical image analyzer (MYN) products applied to mammography breast cancer, ultrasound breast lesions, radiograph lung nodules, and radiograph dental caries detection -- products that were reclassified in 2024 from class III devices to class II devices with special controls.
- Nine radiological computer-assisted triage and notification software (QAS) products.
- 11 medical image management and processing system (LLZ) products, which are eligible for the third-party review program as updated on July 14.
Also, "to support transparency in the use of modern AI technologies, the FDA will explore methods to identify and tag medical devices that incorporate foundation models encompassing a wide range of AI systems, from large language models (LLMs) to multimodal architectures," the agency noted.
Review the full AI-enabled medical device update here.