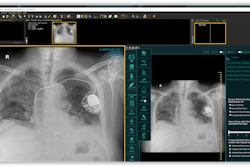
The U.S. Food and Drug Administration (FDA) has cleared Carpl.ai's web-based PACS and radiology workflow management device.
The approval of the enterprise imaging AI platform "opens the door for widespread adoption of AI solutions through a platform approach," said Carpl.ai CEO Vidur Mahajan.
Carpl.ai's system offers radiologists and other physicians a simplified process for selecting, implementing, and procuring third-party AI models that have been cleared by the FDA. The clearance includes Carpl.ai's universal AI viewer which also allows radiologists to use multiple AI applications through a single user interface, according to the firm.
The company said its platform features over 110 AI applications from more than 50 vendors to offer a comprehensive AI marketplace. The platform can be integrated into any major PACS vendor, and hosted on-premise, on private cloud, or on any major cloud provider, it said. The company also noted the platform offers the benefit of "zero incremental costs for AI pilots" and suggested the platform will facilitate rapid scaling of AI use cases and faster time-to-value for healthcare providers.