Nancy Cappello, PhD, was shocked when she received a diagnosis of breast cancer in February 2004. The Connecticut resident ate right, exercised, and had annual mammograms -- in fact, she'd received a negative mammogram just two months prior. But unbeknownst to Cappello, she was a member of an unlucky club of women -- those with dense breast tissue, in whom mammography has less than a 50% chance of catching breast cancer.


In Cappello's case, she underwent the full battery of treatment, including mastectomy, chemotherapy, radiation therapy, and breast reconstruction, and her cancer is currently in remission. She channeled her frustration over her missed diagnosis into political activism, forming the awareness group Are You Dense and helping to pass a Connecticut law in October 2009 that requires physicians to inform patients of their breast density status.
Cappello's story highlights the need for more advanced imaging tools to penetrate the conundrum of dense breast tissue. While ultrasound and breast MRI have won some converts (in Cappello's case, ultrasound helped identify her tumor), other imaging researchers see potential in newer technologies just now arriving at breast care centers.
A tarnished gold standard
Though mammography remains the gold standard for screening women in the general population, the modality faces limitations in women with dense breast tissue, according to Wendie Berg, MD, PhD, a breast imaging radiologist in Lutherville, MD.
"We know that dense breast tissue hides cancer: At least half of cancers present are not identified with mammography in women with dense breast tissue," Berg said. These tumors are similar in density to surrounding tissue, resulting in decreased contrast and obscured visualization of lesions. What's more, there's no way to tell whether a woman has dense breast tissue until she's already been imaged with mammography.
The American College of Radiology (ACR) has developed a widely accepted BI-RADS classification system comprised of four breast density categories:

- Almost entirely fat
- Scattered fibroglandular density
- Heterogeneously dense
- Extremely dense
The latter categories are responsible for the majority of cancers that remain undetected. In fact, a study presented at the 2010 American Association for Cancer Research (AACR) meeting revealed that women with a breast density of at least 75% were four to five times more likely to develop breast cancer than women with little or no density.
Ultrasound has been proffered as a solution to the dense breast conundrum and, indeed, it does improve the performance of mammography in this population of women. For example, according to results from the American College of Radiology Imaging Network (ACRIN) 6666 trial reported in 2008, researchers found that mammography's sensitivity in detecting those cancers was only 50%. However, when ultrasound was added to mammography, the researchers were able to increase sensitivity to 78%, suggesting ultrasound might be a useful supplemental tool in identifying breast cancer for this population.
But ultrasound isn't necessarily an ideal solution: The technique is highly operator-dependent and requires a high level of expertise to perform. This has led some breast imaging specialists to seek out alternative modalities.
Alternative approaches
To begin with, digital mammography has been shown to be more effective for women with dense breast tissue, as well as for women who are peri- or premenopausal (generally women younger than 50), according to the ACRIN Digital Mammographic Imaging Screening Trial (DMIST) reported in 2005.
Besides digital mammography, ultrasound, MRI, positron emission mammography (PEM), and molecular breast imaging techniques are being used to meet the imaging challenges posed by dense breast tissue.
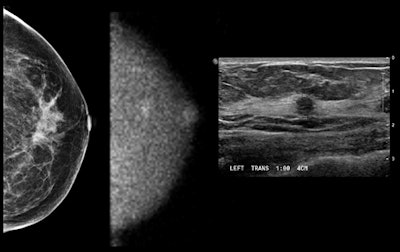
Images show (from left to right) mammogram (craniocaudal view -- read as negative), MBI study (craniocaudal view), and ultrasound study. Patient had an approximately 6-mm invasive cancer of the breast, which is seen on MBI and confirmed by ultrasound and pathology. Patient was a participant in a Mayo Clinic screening study comparing mammography and MBI in women with dense breast tissue. The study was performed using 148 MBq of technetium-99m sestamibi and a 10-minute view. Image courtesy of Michael O'Connor, PhD.
Ultrasound
In current usage, ultrasound provides confirmation of suspicious areas and offers guidance for core needle biopsy procedures. Currently, ultrasound's use as a screening modality is not supported.
Although ultrasound can increase absolute rates of cancer detection 30% above mammography alone, as mentioned, limiting factors of the technology include its operator-dependent nature, the expertise required to perform these time-consuming studies, a shortage of sonographers adequately trained to perform the studies, and low reimbursement rates. Typical Medicare payments average $85 to $120, which is insufficient to fully cover the cost of a breast scan.
False positives generated with ultrasound are also a concern, according to Donna Plecha, MD, an assistant professor and director of breast imaging at University Hospitals of Cleveland. This results in an increased number of biopsies performed on benign lesions, leading to both increased expense and higher anxiety for patients.
Breast MRI
This modality has been recommended for screening use in some high-risk women, and it provides further definition of tumor mass and shape to inform surgical interventions. It is probably too costly to be used for mass screening, however, and like ultrasound it can lead to a large number of false positives that have to be followed up.Breast MRI has other drawbacks, according to Berg. Gadolinium MRI contrast must be injected, and scans require approximately 30 minutes to complete. Some women are not candidates for MRI because they are claustrophobic or have metal implants, and there is a shortage of sonographers adequately trained to perform the studies.
Nuclear breast imaging
With all these techniques, concern about the amount of radioactive tracer required often drives the decision as to how they are used: screening versus diagnostic workup and treatment management.
PEM
Positron emission mammography is a breast-specific PET exam that uses FDG to provide additional information about tumor size and shape.
Plecha describes PEM as an FDG-based scan specifically designed for breast imaging, with smaller detectors than whole-body PET. Its primary application is for use in women who already have diagnosed breast cancer. PEM is not indicated for screening.
In research to appear in the December issue of Radiology, Berg reports on the results of a study of 388 women that compared PEM to MRI. The study found that PEM shows increased precision at identifying benign and cancerous lesions, which helped to reduce unnecessary biopsies. Another benefit PEM holds over MRI is that the scans are easier to interpret, with only 12 images of the breast viewed in two projections for a total of 24 images, compared with 1,000 to 2,000 images produced by a typical MRI.
Issues of radiation dose required for PEM are currently being addressed, with preliminary research suggesting that dose can be cut by as much as 70%, to 111 MBq of FDG, as reported at the American Association of Physicists in Medicine (AAPM) annual meeting in August 2010.
Naviscan of San Diego has commercialized the PEM technology.
BSGI
Breast-specific gamma imaging employs a gamma camera to detect uptake of Tc-99m sestamibi in women with suspicious areas in breast tissue.BSGI uses a single-head gamma camera to detect "hot spots" of abnormally increased metabolic activity found in breast cancer lesions. The original question posed about this technology was whether it was sensitive enough to replace other tools such as MRI or ultrasound, and initial studies suggest it meets necessary sensitivity thresholds.
Dilon Diagnostics of Newport News, VA, has commercialized the BSGI technology.
MBI
Molecular breast imaging employs a gamma camera with one or two semiconductor-based CZT detectors to image the breast after the patient has been injected with Tc-99m sestamibi. The technology has been the focus of researchers at the Mayo Clinic in Rochester, MN.
The first indication for MBI is for the workup of women with suspicious breast lesions found on mammography. But Mayo Clinic researchers are also investigating the feasibility of using the system for screening purposes, primarily in women with dense breast tissue.
Gamma Medica of Northridge, CA, has licensed and commercialized the MBI technology developed at the Mayo Clinic.
MBI's potential
Michael O'Connor, PhD, a professor of radiologic physics at the Mayo Clinic, was instrumental in developing the system. In an initial study of 1,000 women, the system detected three times as many cancers as mammography. The caveat was that the exams used a typical sestamibi dose of 740 MBq, which means the test would not be feasible as a screening technique on an annual or biennial basis. The researchers have since made enhancements to the MBI equipment to enable a reduction in dose to 74 to 148 MBq, resulting in a radiation dose comparable to mammography (see "Radiation dose in women's imaging: Are we scared yet?").

"Right now it's used as a secondary diagnostic tool, but if you are looking at a screening procedure every year or two for 40 years of a woman's life, you must optimize the dose," O'Connor explained. "You would not look at this technology to replace mammography for women who do not have dense breasts, because for them mammography does very well."
Another concern the team has worked to address involves workflow challenges. Enhancements to the system are designed to reduce scanning time from 40 minutes to 25. O'Connor noted that the scans are quick to read because they have only four to eight images.
A comparison of MBI to MRI reveals the cost differential between the technologies, with a typical MRI scan costing $1,100, while MBI is projected to be billed in the $400 to $500 range.
What does the future hold for MBI?
The Mayo Clinic team is exploring the development of a system that combines MBI with an anatomical modality such as ultrasound to enable biopsy while the patient remains in the same position. "We'd like to be able to have the radiologist perform the biopsy with ultrasound guidance while the woman is in the MBI machine," O'Connor said.
Plecha suggested that a system of tailored screening practices that analyzes factors such as breast density, family history, and lifetime risk of breast cancer may provide guidance as to the effective use of all breast imaging techniques. Individual circumstances would inform which imaging technique was most appropriate for a particular patient.
Even with the workflow issues produced by longer scan times of molecular imaging, not all patients would need those exams, so the effect on overall department workflow could be manageable, Plecha noted. She suggested using mammography technologists to position women for the MBI exam, because they possess expertise in this area.
As the imaging community searches for better tools to deal with dense breast tissue, efforts to raise awareness of the problem among women continue. Cappello of Are You Dense has continued her advocacy campaign, and her group is now working with women outside Connecticut, forming a network called the Density Education National Survivors' Effort (DENSE), to lobby for the passage of legislation like the Connecticut law in other states. Bills are being prepared for introduction in at least five states, as well as at the federal level.
"What amazes me is that I hear from radiologists and other physicians [every week] who want to do what we are doing in Connecticut, and are sold on informing women of their breast density," Cappello said.
By Cheryl Hall Harris
AuntMinnie.com contributing writer
September 14, 2010
Copyright © 2010 AuntMinnie.com


















