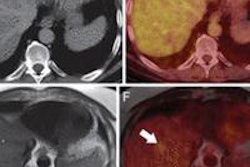
The U.S. Food and Drug Administration (FDA) on September 20 issued a final rule that implements its unique device identification (UDI) system.
The UDI system will provide a consistent way to identify medical devices, and it will improve the reporting of medical device adverse events, according to the agency.
The system consists of two core components, the first being a unique number assigned by device manufacturers to the version or model of a device, called a unique device identifier. This identifier will include production-specific information such as the product's lot or batch number, expiration date, and manufacturing date when that information appears on the label.
The second component is a publicly searchable database that the FDA will administer, called the Global Unique Device Identification Database (GUDID), which will serve as a reference catalogue for every device with an identifier. The FDA said that no identifying patient information will be stored in the database.
The UDI system will be implemented in a phased approach, focusing first on high-risk medical devices. Many low-risk devices will be exempt from some or all of the requirements in the final rule.
The FDA said that high-risk medical devices (class III) must carry unique device identifiers on their label and packaging within one year, and this number and corresponding device information must be submitted to the new database. Manufacturers will have three years to act for most class II (moderate-risk) devices, while manufacturers of class I devices not exempt from UDI requirements will have five years to act.
The FDA has published a draft guidance for manufacturers outlining how to submit information to the database.