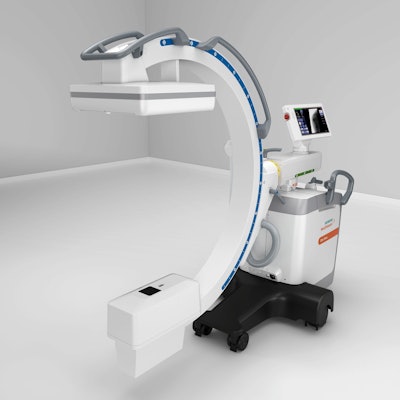
The U.S. Food and Drug Administration (FDA) has cleared the Cios Flow mobile C-arm from Siemens Healthineers.
Designed for multiple uses in operating rooms, Cios Flow can be used in a variety of clinical settings, including orthopedics, trauma surgery, spinal surgery, vascular surgery, and pain therapy, and it aims to enable more patient-friendly surgical interventions.
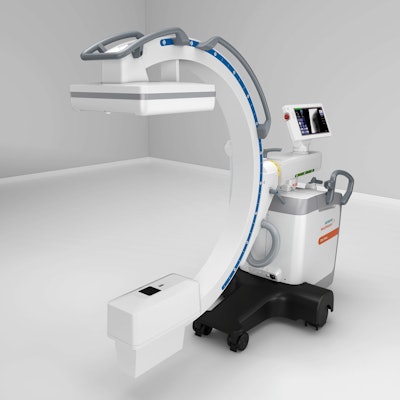 The Cios Flow mobile C-arm. Image courtesy of Siemens Healthineers.
The Cios Flow mobile C-arm. Image courtesy of Siemens Healthineers.Siemens unveiled Cios Flow at the RSNA 2020 virtual meeting. The company highlighted the low weight, maneuverability, and touch-gesture interface of the C-arm. Cios Flow also includes a function called SpotAdapt that helps users visualize areas of challenging anatomy by allowing them to tap an area on a preview image of the user interface; the technique automatically optimizes parameters such as brightness and contrast, according to the company.