
Editor's note: As part of the celebration of AuntMinnie.com's upcoming 25th anniversary, we're presenting 25 for 25 -- a series featuring our most popular content for each of the last 25 years. New articles will be published each Monday until our official anniversary at RSNA 2024. In 2007, our top article offered a deep dive into the association of nephrogenic systemic fibrosis (NSF) with gadolinium-based contrast agents.
Imagine going in for a diagnostic MRI scan and coming out with a progressive, irreversible, and potentially fatal disease. That's what has happened to hundreds of people worldwide who have developed nephrogenic systemic fibrosis (NSF), an acquired disorder in renal patients that's been classified only since 2000.
Short of a kidney transplant, NSF is difficult to manage. But grappling with NSF after the fact may be the least troubling issue as healthcare experts scramble to figure out why some patients develop NSF -- and what they can do to prevent NSF from occurring in the first place. It certainly doesn't help matters that the disease has been linked to gadolinium-based contrast agents, a ubiquitous tool in the MR armamentarium.
In 2006 the U.S. Centers for Disease Control and Prevention (CDC) published the first U.S. report of NSF in 33 dialysis patients at a St. Louis hospital. Later in the year, a Food and Drug Administration (FDA) advisory was released, citing 25 more NSF cases in Denmark and Austria.
Since then, reports on NFS and its connection to gadolinium have been pouring in:
- At the 2007 European Congress of Radiology (ECR) meeting in Vienna, Scottish radiologists reported on NSF episodes in the whole renal failure population of western Scotland. They found 14 cases (0.77%) of confirmed NSF among 1,826 patients. Of these 14 cases, 13 patients had received gadolinium contrast.
- A study by Phillip Kuo, MD, and colleagues at Yale University in New Haven, CT, parsed an international registry containing the records of 215 patients with NSF and found that more than 95% of those patients received gadolinium chelate within three months of disease onset (Radiology, March 2007, Vol. 242:3, pp. 647-649).
- New guidelines on MR safety from the American College of Radiology (ACR) specifically addressed NSF, calling gadolinium administration "a necessary factor in the development of NSF at this time" (American Journal of Roentgenology, March 1, 2007).
- A study out of Loma Linda University Medical Center in Loma Linda, CA, concluded that the odds ratio for developing NSF after gadodiamide exposure was 22.3. The authors also noted that "the onset of first reported cases of NSF in 1997 coincided closely with the rapid rise in the use of high-dose gadolinium injection for MRI and MR angiography (MRA) in the last decade" (AJR, February 2007, Vol. 188:2, pp. 586-592).
- Authors of another paper, this time from the University of Wisconsin in Madison, found that in their patient population, those who developed NSF had a significantly decreased estimated glomerular filtration rate, more proinflammatory events, and underwent more contrast-enhanced MR examinations (Radiology, January 31, 2007).
- In a pilot investigation, dermatologists at the Denver-based University of Colorado Health Sciences Center detected gadolinium in the tissue of patients with NSF, which they called "supportive of an epidemiologic association between exposure to gadolinium-containing contrast material and development of disease (Journal of the American Academy of Dermatology, January 2007, Vol. 56:1, pp. 21-26).
Last week, attorneys for a Cleveland man announced they had filed suit against a gadolinium manufacturer, claiming that he developed NSF from an injection of gadolinium MRI contrast. In addition, the law offices of Jim Sokolove in Newton, MA, have begun running national TV ads recruiting NSF patients as potential clients for a class-action lawsuit.
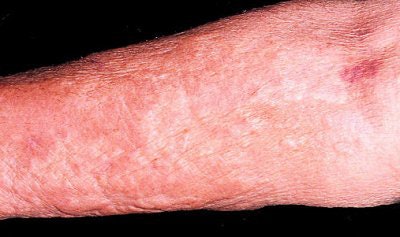 |
| Extensive thickening of the skin, often associated with brawny hyperpigmentation and, in some cases, distinct papules and subcutaneous nodules. Cowper SE. Nephrogenic Fibrosing Dermopathy [NFD/NSF Web site]. 2001-2007. Available at www.icnfdr.org. Accessed 3/5/2007. |
Leonard Berlin, MD, chairman of the department of radiology at Rush North Shore Medical Center in Skokie, IL, told AuntMinnie.com the NSF situation has the potential to rival mammography malpractice.
"In the Ohio case, they're just suing the manufacturer, but that's a short-term thing. You know the next few suits will involve the hospital and the radiologists. It's a malpractice pothole, and a lot of us are going to fall right into it, I'm afraid," Berlin said. "Some lawyers know more about NSF now than radiologists do, which doesn't help the situation."
Clearly, a major problem is at hand. How are imaging experts and others handling NSF? AuntMinnie.com sought answers from many of the people who are involved in solving the NSF riddle.
The chelate connection
NSF involves a systemic restructuring of tissues. Often, it appears as thickened skin so rigid that makes it difficult to bend the joints. As a result, NSF was originally called nephrogenic fibrosing dermopathy (NFD), but underwent a name change to reflect the systemic nature of the disorder, which can include fibrosis of the skeletal muscle, bone, lungs, pleura, pericardium, myocardium, kidneys, muscle, bone, testes, and dura.
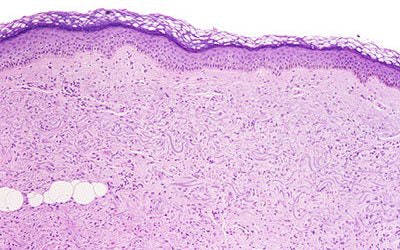 |
| (Above) The dermis demonstrates haphazardly arranged collagen bundles and a strikingly increased number of spindled and plump fibroblast-like cells. (Below) Mucin is frequently present and demonstrable by the colloidal iron alcian blue (pH 2.5) staining methods. Cowper SE. Nephrogenic Fibrosing Dermopathy [NFD/NSF Web site]. 2001-2007. Available at www.icnfdr.org. Accessed 3/5/2007. |
 |
The FDA's first advisory in early 2006 stated that "worldwide, there are approximately 200 reports of NSF," with a December update of "90 patients with moderate to end-stage kidney disease who developed NSF after they had an MRI or MRA with a gadolinium-based contrast agent." The FDA asked physicians to use alternative methods to image patients with moderate to end-stage renal disease whenever possible. Gadolinium agents are approved for MRI but not for MRA, although they often are used off-label for MRA.
Shawn Cowper, MD, an assistant professor of dermatology and pathology at Yale University School of Medicine in New Haven, CT, was the first to identify the condition in 2000 (Lancet, September 16, 2006, Vol. 356: 9234, pp. 1000-1001).
Cowper founded and continues to run the International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR) website. He also maintains the NSF Registry at Yale, which contains information on about 230 cases that he has seen personally, 95% of which involve exposure to gadolinium.
"I think that represents a small fraction of how many cases there actually are. There are easily hundreds, and possibly over a thousand. Many aren't even suspected at this point," Cowper told AuntMinnie.com.
Gadolinium by itself is toxic so all FDA-approved MRI contrast agents minimize toxicity by binding the gadolinium to protective chelates. Gadolinium is renally excreted. In functional kidneys, this gadolinium chelate compound leaves the body entirely within hours.
In patients with renal insufficiency, particularly dialysis-dependent ones, the contrast agent remains in the body much longer, sometimes never leaving entirely. Over time, the gadolinium detaches from the chelates and circulates freely in the body, eventually resulting in fibrosis. While dialysis helps somewhat, it doesn't eliminate all the gadolinium.
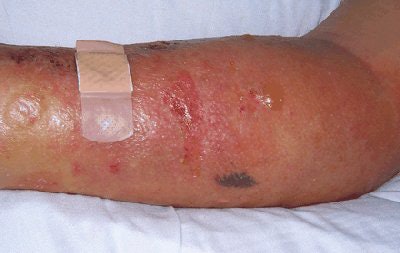 |
| (Above) Patient with NSF presented with area of edema, induration, and erythema on the forearm. (Below) Histopathologic photomicrographs show markedly increase cellularity with spindle-shaped fibrocytes and mucin with thickened collagen bundles that infiltrate deeply, extending into and widening the septa of the subcutaneous fat. (Hematoxylin-eosin stain; original magnification for c, x10; for d, x60.) |
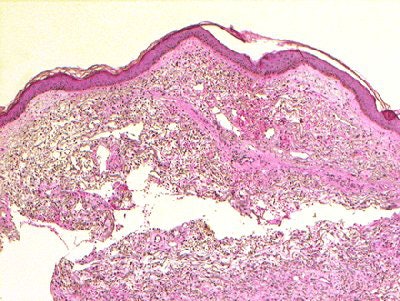 |
| Figure 1a,c. Sadowski EA, Bennett LK, Chan MR, et al. "Nephrogenic Systemic Fibrosis: Risk Factors and Incidence Estimation," Radiology 2007; 243(1):148-157. |
"The best studies show that after three dialysis sessions, you may get 99% of contrast out, but there's always some left behind," Cowper said. "In one of our studies, we took tissue from patients with NSF and did electron microscopy with x-ray dispersion analysis to try to determine whether gadolinium was actually in the tissue that was biopsied. It was found, and in some cases it was found in the skin 11 months out" (Journal of the American Academy of Dermatology, January 2007, Vol. 56:1, pp. 21-26; Radiology, January 31, 2007).
The upshot is that "the gadolinium is getting outside of the vasculature by mechanisms that we don't entirely know at this point, and perhaps then being undialyzable," Cowper explained. "Perhaps it comes unbound from the chelate and then rebinds to whatever anions are available -- proteins or phosphate," in a chemical reaction known as transmetalation.
But exactly how fibrosis is initiated remains a mystery. "We've known for several years that a white blood cell of bone marrow origin called a circulating fibrocyte is involved with this disease," Cowper said. Circulating fibrocytes have the ability to make collagen, and play a part in normal wound healing.
With NSF, fibrocytes "seem to be responding to gadolinium that has been deposited outside of the vascular space," according to Cowper. "They may respond to vascular injury (i.e., the threading of catheters or other surgery) by going to the site and forming collagen inappropriately," he said.
"They also have a capacity to phagocytose particles, so the cells are somehow engulfing the gadolinium," Cowper added. "And what that does is anybody's guess. Maybe the cells die as a result of that phagocytosis, and as a result of dying, maybe other cytokines are formed that perpetuate the disease."
For postreaction patients, various therapies are being tested, including oral steroids, synthetic vitamin D3 to slow skin growth, white blood cell radiation treatment to soften plaque, and plasmapheresis for blood purification.
It would be unreasonable to rule out other factors that could play a role in NSF. Erythropoietin is one possibility, said Elizabeth Sadowski, MD, of the University of Wisconsin. Both gadolinium for MRA and erythropoietin, a red blood cell booster to combat anemia in kidney disease patients, began widespread use at the same time (around 1997), she noted.
Marcia Javitt, MD, section head of body MRI and genitourinary radiology at Walter Reed Army Medical Center in Washington, DC, reserved judgment and would like to see "an absolute, demonstrated, cause-and-effect" relationship between gadolinium and NSF.
"Ask yourself: How many injections of gadolinium have been given worldwide? It has to be millions," she explained. One report stated that between the years 2000 and 2006, there were about 40 million gadolinium doses administered in the U.S. (Diagnostic and Interventional Radiology, December 2006, Vol. 12:4, pp. 161-162).
"You're talking about reported NSF cases in the hundreds. So that doesn't add to my decision-making in any way," Javitt said. "NSF is a pretty debilitating, horrible disease. Clearly there's some inflammatory process going on. We smell smoke, and we're looking for the fire."
Making careful choices
Fortunately, the imaging community has taken the old "ounce of prevention" adage to heart by proposing and adopting risk management strategies for performing gadolinium-enhanced MRI on renal patients.
This sooner-rather-than-later approach is pretty much mandatory as there aren't many alternative imaging options for renal patients, who typically have serious comorbidities, including diabetes and cardiac problems. Imaging is generally required for organs other than the kidneys and MR is the first-line imaging exam.
Substitutions for gadolinium and MR are few and far between. An iodinated contrast agent would be required for x-ray exams but is contraindicated in renal patients, especially those with high serum creatinine (SCr) levels.
According to guidelines set out by the European Society of Urogenital Radiology (ESUR), low-osmolar contrast agents are a possibility, although there may still be a difference between the types of low-osmolar contrast agents. "It is ... clear that all contrast agents can cause nephropathy in the patients with risk factors," warned ESUR's contrast media safety committee (Current Opinion in Urology, January 2007, Vol. 17:1, pp. 70-76).
One of the more controversial aspects about the potential NSF-gadolinium connection is whether certain formulations of gadolinium are more likely to lead to NSF. In its December advisory, the FDA lists all five agents currently approved for use with MRI:
- Omniscan (gadodiamide), GE Healthcare, Chalfont St. Giles, U.K.
- OptiMark (gadoversetamide), Mallinckrodt, Hazelwood, MO
- Magnevist (gadopentetate DTPA), Berlex, Montville, NJ
- ProHance (gadoteridol), Bracco Diagnostics, Princeton, NJ
- MultiHance (gadobenate dimeglumine), Bracco
The FDA stated that while NSF cases have been linked to only three of the five products, the agency believes that there is the potential for the disease to occur with any of the agents.
In the Scottish study, the overwhelming majority (89.5% of all gadolinium-enhanced studies) of MRI studies used Omniscan. The ACR MR safety guidelines cited a Danish Medical Agency report from Denmark and Austria in which 5% of patients who underwent MRI with Omniscan were eventually diagnosed with NSF. The group in Wisconsin found a 5% incidence of NSF among high-risk patients who received Omniscan. But in the Yale study, multiple agents were involved in NSF cases, including Omniscan, Magnevist, and Optimark.
How to pick the "right" gadolinium-based contrast agent? Emanuel Kanal, MD, who leads the ACR Blue Ribbon Panel on MR Safety and is the director of magnetic resonance services at the University of Pittsburgh Medical Center in Pennsylvania, suggested avoiding Omniscan altogether.
"All the gadolinium agents have the same general effect," said Kanal in an interview with AuntMinnie.com. "The differences are in how tightly they bind to the chelate. Omniscan binds least strongly."
GE Healthcare, the maker of Omniscan, is quick to emphasize that "a causal relationship between NFD/NSF and these agents has not been established" ("Omniscan Safety Update," June 6, 2006).
Donald Black, MD, global head of research and development for medical diagnostics for GE Healthcare, told AuntMinnie.com: "The bottom line is that there had been only two cases where patients weren't in moderate to severe renal failure; both of them had acute renal failure at the time. We believe (that) there's a lot of speculation, rather than evidence, that NSF cases are associated with Omniscan in mild renal impairment."
"We've always had on our label that patients with renal impairment should be monitored closely with our drug, and we're strengthening our label to further educate patients, because that's our concern," Black added.
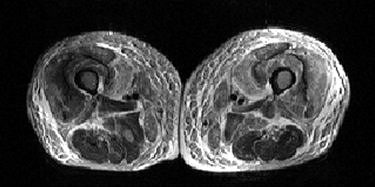 |
| A 59-year-old man with nephrogenic systemic fibrosis (NSF) with both skeletal muscle and skin findings. Axial T1- and fat-suppressed T2-weighted images of thighs show symmetric skin thickening and edema in medial thighs. There is also marked edema in subcutaneous fat, intermuscular fascia, and thigh muscles with some sparing of posterior thigh muscles. Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, and Kirk GA, "Gadodiamide-Associated Nephrogenic Systemic Fibrosis: Why Radiologists Should Be Concerned" (AJR 2007; 188:586-592). |
Sadowski led the University of Wisconsin study that reported a 5% NSF incidence rate in that patient population. She told AuntMinnie.com that it was not a foregone conclusion that gadolinium was solely to blame for NSF. Still, the imaging unit at the university did switch from using Omniscan to using MultiHance on a regular basis.
At Walter Reed there haven't been any cases of gadolinium-related NSF, although Omniscan was never used by the imaging department, Javitt said. She offered some other contrast materials for consideration: a ferumoxides-based one (Feridex, Berlex Imaging, Wayne, NJ) that she helped develop and which the FDA has approved for MRI of the liver, and mangafodipir (Teslascan, GE Healthcare), which is available in Europe (Radiology, August 2003, Vol. 228:2, pp. 457-464; Cancer Research, January 1, 2006, Vol. 66:1, p. 598).
Yes to prescreening; no to standing orders
Perhaps the key is not which gadolinium-based agent is used, but how it's applied. For all contrast media except for Omniscan, Kanal suggested that people "use as little as possible, such as a half dose."
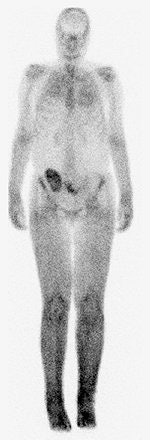 |
| A 30-year-old woman with nephrogenic systemic fibrosis with predominant skin finding. Anteroposterior and lateral Tc-99m HDP delayed bone scan shows extensive symmetric skin uptake in lower extremities and distal upper extremities. Uptake is also seen in calf muscles, Achilles tendon, and chest wall. Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, and Kirk GA, "Gadodiamide-Associated Nephrogenic Systemic Fibrosis: Why Radiologists Should Be Concerned" (AJR 2007; 188:586-592). |
Sadowski spoke in favor of additional screening, prior to any gadolinium-enhanced MR exam: "We screen all the inpatients, and if they have estimated (glomerular filtration rate or eGFR) of less than 60, we look to see if they have any concomitant factors, such as severe infection, sepsis, pneumonia, meningitis, major surgery such as coronary bypass or transplantation, or vascular thromboses such as pulmonary embolism or renal artery thrombosis." If they flunk that eGFR test, "we avoid administering any gadolinium contrast agents to them," she noted.
Outpatients at Sadowski's facility receive MultiHance regardless of their eGFR. In all cases, the hospital obtains verbal informed consent from patients receiving contrast. After the MR scan, patients are followed closely for several months to watch for NSF symptoms, which can occur days or weeks later.
Sadowski said that MR can be avoided altogether by using ultrasound, CT, or molecular imaging. For patients who are on dialysis, "iodine can be used freely as long as the patient gets dialysis after the contrast is given," she said.
The "ACR Guidance Document for Safe MR Practices: 2007" stated that "for all patients with stage 2, 4, or 5 kidney disease or those with acute kidney injury, it is recommended that one consider refraining from administering any (gadolinium-based contrast agent) unless a risk-benefit assessment for that particular patient indicates that the benefit of doing so clearly outweighs the potential risk(s)."
"The ACR and the FDA recommend immediate hemodialysis directly from the MR scanner," Kanal said, plus another dialysis session within 24 hours if it's clinically safe to do so. "Considering that transmetalation is the most likely theory of what's going on, and the release of free gadolinium, it's extremely important to begin getting rid of these agents immediately after you no longer need them, which is the second you finish your MR exam."
In addition, patients "need to provide informed consent," Kanal advised. "There also needs to be an explicit order signed by a radiologist -- not a referring physician -- that Mrs. Jones is to receive a certain amount of a specifically named brand of (gadolinium-based contrast agent) for this specific MR examination. It can't be a generic or a standing order. That way, the radiologist will participate in the responsibility."
But Berlin cautioned that the lack of definitive answers in NSF may make informed consent a moot point.
"What are we going to inform the patient (of)? We have limited information ourselves. So basically, it's going to fall back on the patient asking the doctor: 'What do you think -- is it safe or not?' And (the answer) is really going to rest with the radiologist or the referring physician," Berlin said. A reassurance from the physician may be enough to get the patient to sign the consent form, but should something go wrong, the patient is likely to cry foul, he added.
In a worst-case scenario, gadolinium-based contrast agents could wind up in the same unenviable position as other pharmaceuticals. "I personally think we're going to be in for another Vioxx situation," Berlin said. "You have these hundreds, if not thousands, of lawsuits around the country for Vioxx," referring to the anti-inflammatory drug that was linked to cardiovascular risks, and eventually pulled from the market by its manufacturer.
In the meantime, a radiologist's best bet is to proceed with extreme caution when considering gadolinium-enhanced MR in renal cases. "We need to focus on patient safety, and try to create guidelines for management that are based on objective fact, not conjecture," Javitt said. "If you render appropriate patient care, that's the best protection."
Admitting that you don't have all the answers may be required, Berlin advised. "You have to say to the patient you don't really understand the incidence of this thing; that it appears to be low incidence (and) you'll use the safest contrast that you can," he said. "You do the best you can, but some people are going to end up being sued. Even the guidelines are subject to change. It's all evolving, and I think we're going to see a lot of (NSF) patients over the next six months."



















