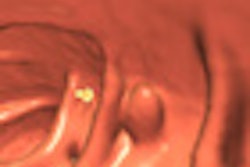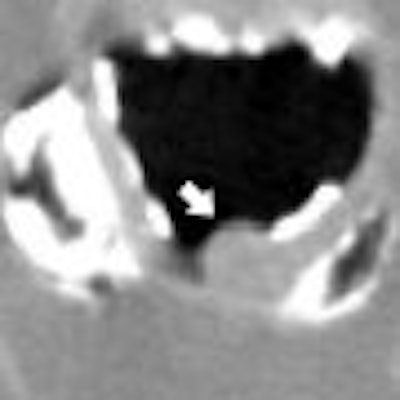
The emergence of preparation-independent computer-aided detection (CAD) for virtual colonoscopy (VC or CT colonography [CTC]) is a sure sign of the growing sophistication of the software.
|
CAD under the hood How does a CAD system manage to detect polyps and separate them from other findings in the presence of widely varying distribution of fecal matter? First, the polyp detection scheme must work not only in the presence of tagged fluid but also tagged feces, explained principal investigator Janne Näppi, Ph.D., in an interview with AuntMinnie.com. Last year Näppi and his co-investigator Dr. Hiro Yoshida, both from Harvard Medical School and Massachusetts General Hospital in Boston, showed how a "combination of densitometric subtraction of tagged materials and volumetric shape-based polyp detection can be used to effectively implement such a scheme," Näppi said of the study published in Academic Radiology (March 2007, Vol. 14:3, pp. 287-300). Another challenge is finding a way to identify solid feces very precisely, because any unidentified feces could be detected as polyps or masses by the CAD system, Näppi said. "In our solution, a pseudoenhancement correction method is used to minimize the local distortion of CT values of polyps due to adjacent positive-contrast tagging agents," he said. "The method permits us to use a single CT threshold value (e.g., 100 HU) across cases to identify poorly tagged feces -- without missing polyps whose CT values are artificially elevated to higher values due to pseudoenhancement." This process, which employs an exponential wavefront method to minimize the artificial enhancement, will be described more fully in an upcoming issue of Medical Image Analysis, Näppi noted. Another problem is feces that remain completely untagged, many of which have polyp-like shape and density characteristics, Näppi said. CAD can successfully identify many completely untagged feces by analyzing their connectivity with the colonic wall. In a study Näppi presented at the 2007 RSNA meeting, the group showed that connectivity analysis significantly reduced false-positive CAD detections due to untagged feces. |
An investigational CAD scheme, presented at the 2008 European Congress of Radiology (ECR) in Vienna, successfully analyzed both cleansed and uncleansed patients, tagged and untagged cases, to detect colorectal polyps and distinguish them from fecal material.
Detection sensitivity for clinically significant lesions remained high across all types of bowel preparations, though the number of false-positive detections rose significantly in uncleansed colons.
"Most current CAD systems assume that a colon is cleansed thoroughly by the laxative before the patient is scanned and CAD is applied," said Dr. Hiro Yoshida in his ECR presentation. However, a CAD system that does not assume bowel cleansing is emerging, he said.
Yoshida and his research colleagues, Janne Näppi, Ph.D.; Wenli Cai, Ph.D.; and Dr. Michael Zalis, are from Boston's Harvard Medical School and Massachusetts General Hospital.
The latest iteration of the group's CAD scheme applies a number of innovations designed to mitigate technical problems that arise when analyzing colonic mucosa in the presence of fecal material and potentially confounding anatomic structures.
In a retrospective study aimed at testing the system's capabilities, polyps were detected by using two volumetric shape features, the authors explained. False-positive detections were reduced along with processing time by using a neural network based on volumetric shape and texture features that eliminated unlikely polyp candidates. Finally, a pseudoenhancement correction method was used to minimize local distortion of CT attenuation surrounding contrast tagging agents, and a connectivity analysis algorithm was applied to eliminate detections with insufficient connections to the colon wall.
The need for more robust CAD systems will continue to grow as virtual colonoscopy moves into widespread clinical practice, Yoshida said. Radiologists -- and CAD applications -- will have to deal with a wide range of bowel preparations and minimal-prep protocols yielding different CAD outcomes.
The preparation-independent CAD used in the study could help by adapting automatically to different bowel conditions, he said.
The CAD scheme used in the study employs a fully automated process to convert CTC data into a preparation-independent representation, Yoshida said. This is followed by a polyp-detection step, then false-positive reduction. Finally, the detected polyp candidates are displayed on a CTC reading workstation for review.
The researchers "trained" their CAD scheme using 60 colonoscopy-confirmed polyps in 150 patients from three U.S. centers that had used conventional CTC protocols, including 24-hour or 48-hour bowel preparations from two laxative regimens (group 1), two reduced-laxative regimens (group 2), and three laxative-free regimens (group 3). Cases used to evaluate the system were independent from the training data, he said.

Patient data were acquired on a variety of multislice scanners in the five facilities, with collimation ranging from 0.6 mm to as wide as 5 mm in a few minimal-prep cases, Yoshida explained. There was more variation in the CT parameters among the reduced-preparation and laxative-free groups, he said. Reconstruction intervals varied widely as well, from 0.7 mm to 5.0 mm, and tube current ranged from 28 mAs to 200 mAs.
"We used a total of 292 scans with 146 supine and 146 prone," Yoshida said. The colonoscopy-confirmed findings were broken down by patient group:
- Laxative group: 18 adenomas 6 mm or larger, including seven adenomas 10 mm or larger and 10 adenomas 8 mm or larger
- Reduced-laxative group: 17 adenomas 6 mm or larger, including eight adenomas 10 mm or larger and 12 adenomas 8 mm or larger
- Laxative-free group: 13 adenomas 6 mm or larger, including five adenomas 10 mm or larger and seven adenomas 8 mm or larger
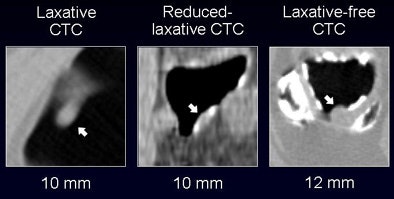
"The CAD yielded per-polyp and per-patient sensitivity of around 90% for polyps 6 mm and larger, with sensitivity figures remaining high for larger polyps," Yoshida said. "Overall sensitivity for polyps larger than 10 mm was 100%, with 2.8 false positives per scan. For polyps 6 mm and larger, sensitivity dropped to 82%, with approximately 4.3 false positives per case."
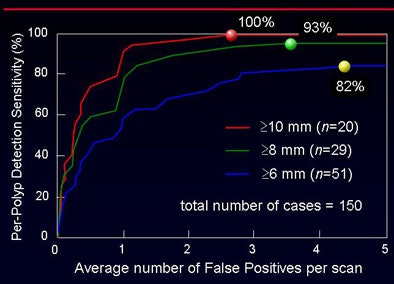
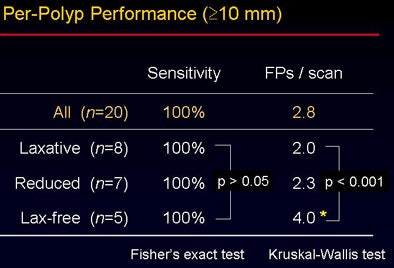
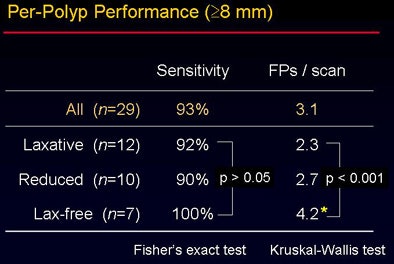
The overall accuracy did not vary significantly between the groups despite the use of three different bowel preparations (p > 0.05). "However, when you look at the false-positive rate, there is a statistically significant difference among the three groups" (p < 0.001), Yoshida said. The sources of false-positive CAD detections were also different among the three groups, as shown in the image below.
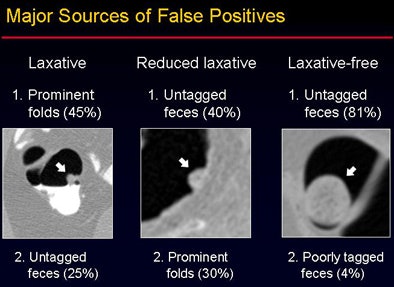
"The performance of laxative-independent CAD was consistent across multiple bowel preparations ranging from full cathartic to noncathartic prep," he concluded, noting that its flexibility could be highly desirable in clinical practice.
By Eric Barnes
AuntMinnie.com staff writer
May 6, 2008
Related Reading
VC CAD beats human readers in multicenter trial data, November 19, 2007
Polyp ranking scheme boosts CAD efficiency, October 1, 2007
Fully automated VC CAD system solves tagging problems, June 25, 2007
VC CAD ramps up detection of medium-sized polyps, March 12, 2007
CAD will be key to VC screening, September 13, 2006
Copyright © 2008 AuntMinnie.com